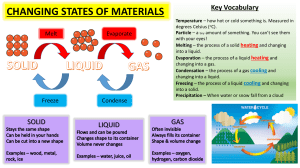
Phase Changes • Physical change of matter from one phase to another due to a transfer of energy. Evaporation • Liquid – gas at surface of a liquid. • Molecules gain KE to become a vapor. Molecules left behind lose KE. • Cooling process – molecules left behind lose KE (cooler). Condensation • Gas – liquid. • Gas molecules ↓KE when collide with cold surface. Condense to liquid phase. • Warming process – KE lost by condensing gas molecules warms the surface they strike. Evaporation-Condensation Rates • Depends upon environment, if moist: – Condensation > evaporation (warming) – Evaporation > condensation (cooling) • Equilibrium – state of balance. Evaporation and condensation occurring at equal rates. Boiling • Liquid – gas beneath the surface of a liquid. • Bubbles of vapor form beneath the surface – rise – break free to the vapor phase. • ↑atm. pressure - ↑boiling pt. • Cooling process – the water is being cooled relative to the ↑temp it would attain otherwise. Because of cooling, it remains 100 °C instead of getting hotter. Freezing • Liquid - solid. • Energy is extracted, molecules slow down and the molecular attraction overcomes the KE. • Warming process – when you make ice cubes, you put liquid water in the freezer. The freezer cools the water, taking energy out – so it must give off energy. Melting • Solid – liquid. • Heat energy is added until KE is greater than the molecular attraction. • Cooling process – the source of energy is the object or material around the stuff that is melting. The source cools as energy goes to the melting object. Sublimation • Solid - gas. • Example: mothballs that “evaporate” without leaving a liquid, and when snow on the ground “evaporates” skipping the liquid phase altogether. Phase Change Graph Phase Change Diagram Temperature °C Heat of vaporization C.P 100 Heat of fusion Condenses Boiling Freezing F.P O M.P Melting Heat Energy B.P Phase Changes Phase Changes From what Phase-to-Phase does change occur Absorb or Release Heat Energy? Cooling or Warming Process Melting solid-liquid Absorb Cooling Freezing liquid-solid Release Warming Vaporization liquid-gas Absorb Cooling Condensation gas-liquid Release Warming Sublimation solid-gas Absorb Cooling Heat of Fusion • Amount of heat needed to change 1g substance from solid – liquid phase. • 80 calories/g for water. • Example: How many calories are needed to change 10g of ice at 0 °C to 10g of water at 0 °C? 800 Heat of Vaporization • Amount of heat needed to change 1g substance from liquid – gas phase. • 540 calories/g for water. • Example: How many calories are needed to change 10g of water at 100 °C to entirely to water vapor? 5400 Sample Problem How much heat is required to change 1g of ice at -20°C to water vapor at 130°C? 1. Q = mc∆t 1g · 0.5 cal/g-C · 20 °C = 10 cal. 2. Hf 1g · 80 cal/g = 80 cal. 3. Q = mc∆t 1g · 1 cal/g-C · 100 °C = 100 cal. 4. Hv 1g · 540 cal/g = 540 cal. 5. Q = mc∆t 1g. · 0.5 cal/g · 30 °C = 15 cal. 745 calories