Polymer Technology Presentation: Materials Science Overview
advertisement

Book: Plastics Materials: John A. Brydson Principles of Polymerization: George Odian Properties of Polymers: D.W. Van Krevelen Polymer Materials: Sukumar Maiti Polymer Technology (MS41004) Prof. Dr. Susanta Banerjee Institute Chair Professor Materials Science Centre IIT Kharagpur 1 Materials and civilisation Stone age (Before 5000 BC) Copper age (5000 - 3000 BC) Bronze age(3000 - 800 BC) Iron age(800 BC - 40 AD) Plastic age ? 2 Types of materials Metal Semiconductors Ceramic Composites Molecular materials 3 Polymer Biomaterials History of polymers (1500 – present) 1500’s British explorers discover ancient Mayan civilization in Central America. The Mayans are assumed to be the among The first to find an application of polymers, as their children Were fond of playing with balls made from local rubber tree 1839 Charls Godyear discovers Vulcanisation . Vulcanised rubber is much is much more durable than its natural part 1917 X-ray crrstallography is invented as a method of analyzing crystal structure. M. Polanyi discover the chemical structure of cellulose. This establishes the fact that polymer unit cells contain Sections of long chain molecules rather than small molecules 1907 The oldest recorded synthetic Plastic is fabricated by Leo Bakeland. Bakelite’s hardness and high heat resistance made it an excellent choice as an electrical insulator and many article 1920 Hermann Staudinger pulished his classic paper “Uber Polymerization”. Demonstrated the existence of macromolecules Nobel Prize in Chemistry in1953 for his discoveries in the field of macromolecular chemistry 1927 Large scale production of PVC resins begins. Widely used today to make plumbing pipe, tiles and bottles. 1930 Polystyrene invented. Material used in video cassettes , toys and other packaging. 1970 Development of liquid crystalline polymer.Common application in electronic devices and Aircraft engines Xydar® LCP is a glass fiber or mineral-filled resin that features excellent flow properties. It can be injection molded into thin-wall components and has outstanding strength at extreme temperatures to 300°C. Xydar® resin isinherently FR, transparent to microwave radiation, and resistant to virtually all chemicals. 1971 Kevelar developed. High strengthmaterial that can withstand temp. upto 300 oC. Used in bullet proof vests, fire proof germents for fire fighting and auto raching. HBA/TPA/BP 1938 W. Carotheror (DuPont ) Invented Nylon, is a common material in clothing and different engineering products 1941 Polyethylene developed. Billions of poundes of LDPE and HDPE is produced today, everything for packaging film, piping to toy 1976 The polymer product industry outstrip steel as the most widely used material per unit volume. We now use more plastic than steel, aluminum and copper combined 5 World consumption of polymers 6 Use of polymers in different sector (European Union) 7 Cost Performance 8 Packaging 9 Sporting Goods 10 Consumer products 11 Aircraft Airbus A380 12 Automotive The 100% plastic car – no longer an imaginary concept !!! 13 Naval Ships 14 Polymers • The earliest synthetic polymer was developed in 1906, called Bakelite. • The development of modern plastics started in 1920s using raw material extracted from coal and petroleum products (Ethylene). Ethylene is called a monomer. • Polymers are long-chain molecules and are formed by polymerization process, linking and cross linking a particular building block (monomer, a unit cell). • The term polymer means many units repeated many times in a chainlike structure. • Most monomers are organic materials, atoms are joined in covalent bonds (electron-sharing) with other atoms such as oxygen, nitrogen, hydrogen, sulfur, chlorine,…. 15 Products from Bakelite 16 Polymer Building Blocks Hydrogen Carbon (key) Oxygen Nitrogen Fluorine Silicon Sulfur Chlorine Phosphorus 17 Monomer to Polymer HOOC-R-COOH HO-Ar-OH CH3-CH3 CH2=CH2 *CH2-CH2* 18 Polymer, Monomer; Structure unit, degree of polymerization Poly- means "many" and -mer means "part" or "segment". Mono means "one". So, monomers are those itty bitty molecules that can join together to make a long polymer chain. Monomer : material employed in the preparation of the polymer. They are functional & minimum functionality is two. Many many many MONOmers make a POLYmer! 19 Structure units are connected to one another in the polymer chain, or polymeric structure, by covalent bonds. Repeat unit: The atoms that make up the backbone of a polymer chain come in a regular order, and this order repeats itself all along the length of the polymer chain. For example, look at polypropylene : CH2 CH CH2 CH CH2 CH CH2 CH CH3 CH3 CH3 CH3 Its backbone chain is made up of just two carbon atoms repeated over and over again. One carbon atom has two hydrogen atoms attached to it, and the other carbon atom has one hydrogen atom and one pendant methyl group (CH3). This is called the repeat structure or the repeat unit. To make things simple, we usually only draw one unit of the repeat structure, like this: CH2 CH CH3 n 20 Another example: styrene monomers join together to make polystyrene: CH2 CH CH2 CH n Styrene monomer Polystyrene repeat unit CH2 CH CH2 CH CH2 CH CH2 CH 21 Degree of polymerization: refers to the number average obtained by dividing the total number of structural units by the total number of molecules. Polymer molecular weight: M = DP x Mo CH2 CH Mo: molecular weight of repeating unit DP: Av. Degree of polymerization CH3 n As for example look at polypropylene If n = 1000, DP is 1000, This means that CH2 propylene unit has repeated 1000 times and Mo for the repeat unit is 42, the molecular weight (M) of the polymer is 42 X 1000 = 42000 g/mol CH CH3 1000 22 Molecular Weight of Polymers Unlike small molecules, polymers are typically a mixture of differently sized molecules. Only an average molecular weight can be defined. • Measuring molecular weight • Size exclusion chromatography • Viscosity • Measurements of average molecular weight (M.W.) • Number average M.W. (Mn): Total weight of all chains divided by no of chains • Weight average M.W. (Mw): Weighted average. Always larger than Mn • Viscosity average M.W. (Mv): Average determined by viscosity measurements. Closer to Mw than Mn Molecular Weight Distribution PDI = Mw/Mn 23 Molecular Weight of Polymers a) Number average moleculare weight (Mn) Mn: This gives you the true average weight Let's say you had the following polymer sample: 2 chains: 1,000,000 Dalton 2,000,000 5 chains: 700,000 Dalton 3,500,000 10 chains: 400,000 Dalton 4,000,000 4 chains: 100,000 Dalton 400,000 2 chains: 50,000 Dalton 100,000 10,000,000 10,000,000/23 = 435,000 Dalton 1 Dalton = 1 g/mole Mn = ∑niMi/∑ni 24 b) Weight Average Molecular Weight (Mw) Mw: Since most of the polymer mass is in the heavier fractions, this gives the average molecular weight of the most abundant polymer fraction by mass. 2,000,000 0.20 1,000,000 200,000 10,000,000 3,500,000 0.35 700,000 245,000 10,000,000 4,000,000 0.40 400,000 160,000 10,000,000 400,000 0.04 100,000 4,000 10,000,000 100,000 0.01 50,000 500 10,000,000 Total 609,500 Mw = ∑niMi2/∑niMi 25 Chemical Structure CH2 Homoplymer- only one monomer (repeating unit) CH n -A – A – A – A – A – A – A – CF2 CF2 CH2 CH CH2 CH2 n CH3 n n Copolymer – more than one monomer CH2 CH2 CH2 CH CH2 CH CH2 CH CH CH2 n CH3 n 26 Copolymers Alternating and random -A–B–A–B–A–B–A–BCH2 CH2 CH2 CH CH2 CH2 CH3 CH2 CH CH2 CH2 CH2 CH CH3 n CH3 Block -A-A-A-A-A-B-B-B-B-B-A-A-A-A-ACH2 CH CH2 CH CH CH2 x CH2 CH y z n -A-A-A-A-A-A-A-B-B-B-B-B-B-B- Copolymers can be used to tailor functionality or generate new phases and behaviors. 27 Copolymers Graft Polymer Science: Vasant Gowariker, N. V. Viswanathan, Jayadev Sreedhar Poly(styrene)-graft-poly(butadiene) B-B-B-B-B-B-B B -A-A-A-A-A-A-A-A-A-A-A-A-A-A-AB B-B-B-B-B-B 28 Various polymer architectures 29 • Homopolymers may not always satisfy all the requirements for various practical applications. • Thus, initially the trend was to develop new polymers from a wide variety of monomers. • For tailoring or modifying the properties of polymers, copolymerization has been used as an effective technique. • However, due to the economic and technical uncertainties associated with synthesizing new polymers, recently efforts were focused on multiphase polymeric systems to obtain materials with improved physical and chemical properties and such considerations were the basis of polymer blends. • Mixing two or more polymers together to produce blends is economically a more attractive way than the copolymerization from the standpoint of commercial applications. • BLENDS: 1. Miscible; 2. Partially miscible; 3. Immiscible (Polymer A & B) Viton CH2=CH2 CF2=CF2 CF2=CF(CF3) 30 A -(A)x-(B)y- 1/Tg(blend) = WA/TgA + WB/TgB B WA= 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 WB= 100, 90, 80, 70, 60, 50, 40, 30. 20, 10, 0 2 Homopolymers, 9 copolymers A B A = PPO, TgA = 210 oC B = PS, TgB = 100 oC Calculate cohesive energy density by group contribution methods (Bondi) Polymer blends of AB By measuring the Tg of the blends (DSC) 31 Books 1. Plastics Materials, J. A. Brydson 2. Properties of Polymers, D. W. Van Krevelen 3. Principles of Polymerization, George Odian 32 What makes polymers different 1. Can bend and twist and get all tangled up • Chain entanglement: Long polymer chains get entangled with each other. The longer a polymer chain is, the more tangled up it can get. • Since the chains are harder to pull out or separate, that can make things made out of polymers stronger. • When the polymer is melted (heated), the chains can flow past each other. • Below the melting point, the chains can move, but only slowly. Thus the plastic is flexible, but cannot be easily stretched. • Below the glass transition point, the chains become locked and the polymer is rigid 33 2. Polymer Chains Stick to Other Polymer Chains (Summation of Intermolecular Forces): • Think of molecules as being like magnets. Some are like very weak magnets, and some are like strong magnets. So, some can be pulled apart easily, but others take a lot more energy to pull them apart. • Polymer chains are like this too, but remember that they're much much longer than molecules. When the chains stick together very strongly, it can be really tough to pull them apart. • If the chains happen to be straight and stiff and all lined up next to each other, it can be REALLY hard to pull them apart. A great example is cellulose in wood. The chains lay next to each other, straight and sticky (like strong magnets). That makes trees (and lots of houses!) strong and tall. 34 3. Polymer Chains Move Slower than Molecules (Time Scale of Motion) • We can see what happening when polymers dissolve in a liquid. Those long chains move around so slowly that they make the solution flow much slower. The longer the chains, the slower the flow. • If we measure how long it takes for a polymer solution to flow through a special tube, we can learn more about how big the polymer chains are. • By the way, the longer it takes to flow, the more viscous (viss-cuss) it is, polymer enhances the viscosity of the medium 35 Natural Classification of polymers Semi synthetic 1. Source Synthetic 2. Structure 3. Mol. force (a) Linear polymer (b) Branched chain polymer (c) Cross–linked polymer 5. Method of polymerization (a) Solution polymerization (b) Melt or bulk polymerization (c) Emulsion polymerization (d) Suspension polymerization (e) Interfacial polymerization (a) Thermoplastic (b) Thermosetting (c) Elastomers (d) Fibres Continuous / Batch 4. Mode of synthesis/ Mechanism of Polymerization (a) Addition polymers/Chain growth polymers (b) Condensation polymers/ Step growth polymers (c) Ring opening polymerizaion (a) Crystalline (b) Semi-crystalline (c) Amorphous 36 1) Based on the source a) Natural polymers: The polymers obtained from nature (plants and animals) are called Natural polymers. Ex. Starch, cellulose, Natural rubbers, proteins, Nucleic acid (DNA, RNA), cotton, silk, wool H2C H2C H C C C C H3C CH2 H3C CH2 H n n trans-1,4-polyisoprene (Plastic, at RT) ciss-1,4-polyisoprene (rubber, at RT) Cellulose (Fiber) a) Synthetic polymers: The polymers which are prepared in the laboratories are called synthetic polymers e.g. Poleythylene, polyvinylchloride (pvc), Nylon, Teflon, Backelite, Terylene etc CH2 CH2 n CF2 CF2 CH2 CH n Cl n 37 2) Based on the structure a) Linear polymer : In linear polymer the monomeric units are linked together to form linear chain. E.g. Polyethylene (HDPE), Nylon, polyester etc. (b) Branched chain polymer : In branched chain polymers the monomeric units are joined to form long chains with side chains (or) Branches of different length. E.g. Glycogen, starch, L.D.P.E. etc 38 (c) Cross–linked polymer: In cross linked polymers the monomer units are cross linked together to form a three dimensional Network. E.g. Bakelite, melamine–formaldehyde Resin polystyrene–Butadiene. 39 3) Based on the molecular forces a) Thermoplastic : Thermoplastics are the polymers which soften on heating and harden on cooling reversibly. E.g. Polyethylene, polysterene, pvc, Teflon, Nylon, sealing wax. Linear or branched polymers which can be softened or melted when heat is applied. Can be molded into any shape with processing techniques such as injection molding or extrusion. CH3 CH2 CH2 CH2 CH n CH3 n O C CH3 O O C n 40 b) Thermosetting: Thermosetting plastics are the polymers which undergo permanent change on heating(irreversible) E.g. Bakelite, Polyester, Polysiloxanes. • Normally are rigid materials. • Network polymers in which chain motion is greatly restricted by a high degree of crosslinking. • Cannot be reshaped once formed Crosslinked (Networked) – chains are connected to other chains 41 c) Elastomers: Elastomers are the polymers which posses elastic character. E.g. Natural rubber Crosslinked (networked) rubbery polymers that can be stretched easily (3-10x original size) Rapidly recover original dimensions when applied stress is released. Low degree of crosslinking H2C CH2 C C H3C H n Amorphous Polymer – Lightly Crosslinked 42 d) Fibres: Fibres are polymers in which the chains are held by intermolecular forces like H–bond, Dipole–dipole interaction. E.g. Nylon, poly Acrylonitrile H2N (CH2)6 NH2 + HOOC (CH ) COOH 2 4 O HN (CH2)6 NH C C(CH2)4 CH2 O C CH C N n n 43 4) Based on the mode of synthesis (mechanism of formation) a) Addition polymers : A polymer formed by direct addition of repeated monomer without the elimination of by product molecules are called Addition polymer. E.g. Polyethylene, polypropylene, pvc, Teflon, orlun, Neoprene, pvp (polyvinyl pyrolidone) n CH2 CH2 CH2 CH2 n b) Condensation polymers: A polymer formed by the condensation of two (or) more monomers with the elimination of simple molecule like H2O, NH3, HCl, alcohol etc. are called condensation polymer.E.g. Polyester, Nylon 6,6, Bakelite, Polycarbonate CH3 HO C O OH + Cl C Cl CH3 O CH3 C O + HCl O C CH3 n O O O C 44 Semicrystalline Thermoplastic 45 Crystalline Example: Polyethylene 46 47 Polymer Microstructure CH2 CH2 Polyolefins with side chains have stereocenters on every other carbon CH3 n CH2 CH3 CH3 CH3 CH3 CH3 CH3 CH3 With so many stereocenters, the stereochemistry can be complex. There are three main stereochemical classifications for polymers. CH CH3 Atactic: random orientation Isotactic: All stereocenters have same orientation Syndiotactic: Alternating stereochemistry 48 49 Polystyrene (PS) Why is this important? Tacticity affects the physical properties Atactic polymers will generally be amorphous and could be soft, flexible materials (Example: PP) Isotactic and syndiotactic polymers will be more crystalline, thus harder and less flexible Polypropylene (PP) is a good example Atactic PP is a low Tg, gooey material Isoatactic PP is high melting (176º), crystalline, tough material that is industrially useful Syndiotactic PP has similar properties, but is very clear. It is harder to synthesize 51 Why Design with Polymers? • Light weight, high strength to weight ratio, particularly when reinforced Density • Relatively low cost compared to metals and composites Cost 52 Why Design with Polymers? Corrosion resistance Low electrical and thermal conductivity, insulator Easily formed into complex shapes, can be formed, casted and joined. Wide choice of appearance, colors and transparencies Performance Cost Adaptability (soft, hard, crosslink etc) 53 Disadvantages of using Polymers Low strength o Low useful temperature range (up to 600 F; 315 oC) Less dimensional stability over period of time (creep effect) Aging effect, hardens and become brittle over time Sensitive to environment, moisture and chemicals Poor machinability 54 55 10 CH2 CH2 25 CH2 CH2 1200 500 15 CH2 CH2 1000 15 CH2 CH2 15 CH2 CH2 1800 30 CH2 CH2 2000 800 20 CH2 CH2 900 Total number of chains = 150 20 CH2 CH2 2200 Av. DP = 10*500+15*1000……./150 =211000/150 = 1406.6 56 Mechanical Properties of Various Plastics Steel: 350 to 1900 MPa Brass: 200 to 850 MPa Aluminum: 100 to 550 MPa 57 Hardener 58 59 Polymer Synthesis There are two major classes of polymer formation mechanisms Addition polymerization: The polymer grows by sequential addition of monomers to a reactive site Chain growth is linear Maximum molecular weight is obtained early in the reaction Step-Growth polymerization: Monomers react together to make small oligomers. Small oligomers make bigger ones, and big oligomers react to give polymers. Chain growth is exponential Maximum molecular weight is obtained late in the reaction 60 Polyaddition Reactions in which monomers combine without the elimination of a small molecule. CH2 CH X CH2 CH n X X = H, CH3, Cl, CN, Ph, COOCH3, COOH Usually involves the breaking of a double bond. 61 Polyaddition with Radicals Initiation – Creation of an active site (free radical). CH2 In R CH RCH2 CH X X Propagation – Growth of polymer chain by addition of a monomer to an active site and the creation of a new active site. n CH2 CH X RCH2 CH X RCH2 CH CH2 CH CH2 CH X X n X 62 O C O -CO2 CH2 CH2 CH2R CH2 R(CH2-CH2)n1-CH2=CH2 + CH3-(CH2-CH2)n2-CH2-CH2R 63 Polyaddition with Radicals Termination – Growth of chain stops. Combination – Two growing chains collide. Disproportionation – A hydrogen atom is added to the end of a growing chain. 64 Addition Polymerization nA A In* In A A A A* Initiation In Propagation A A A A A* n *A A *A A A A A m A A A A m In In A A A A A A A A A A In n n A* A A A A A Combination B A A A A m Chain Transfer New reactive site is produced Disproportionation Termination Reactive site is consumed MW MW 0 100 % conversion A A A A A n k propagation k ter mination m Types of Addition Polymerizations Anionic Ph C3H7 Li n Li+ C4H9 Ph Li+ C4H9 n Ph Ph Ph Radical PhCO2• Ph n Ph PhCO2 n Ph Cationic Ph Cl3Al OH2 PhCO2 Ph Ph n Ph H Ph HOAlCl3 HOAlCl3 H n Ph Ph 66 Common Polyolefins Monomer Ethylene Polymer Polyethylene CH3 H3C n Repeat unit CH3 CH3 n Polypropylene Propylene CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 Ph n Polystyrene Ph Ph Ph Ph Ph Ph Ph Styrene CH3 Cl n Poly(vinyl chloride) Cl Cl Cl Cl Cl Cl Cl Vinyl Chloride F2C CF2 Tetrafluoroethylene F3C Poly(tetrafluoroethylene): Teflon F2 C C F2 F2 C C F2 F2 C C F2 F2 C C nF 2 F2 C C F2 F2 C C F2 CF3 67 Polycondensation Reactions in which small molecules (H2O, HCl) are eliminated when the monomers combine. O n O O O O O + n HN 2 O NH2 O O O HN OH HO NH O O Polyamide acid O H2O N O n O N O n O + (2n-1) H2O 68 O H2N O NH2 O O N N O O O O O O O O O O NH2 N O O N O 69 Principles of polymerization Textbook by George G. Odian O O n HO C C OH O O C + n HO CH2 CH2 OH O C O CH2 CH2 + (2n-1) H 0 O n 2 MF: C10H8O5 = 208 g/mol 70 Step-Growth Polymerization Stage 1 n n Consumption of monomer Stage 2 Combination of small fragments Stage 3 Reaction of oligomers to give high molecular weight polymer 71 Step-Growth Polymerization • Because high polymer does not form until the end of the reaction, high molecular weight polymer is not obtained unless high conversion of monomer is achieved. DP = (1+r)/(1+r-2rp) Where r = NA/NB If there is no stoichiometric Imbalance; NA = NB DP = 1/(1-p) p=1 DP = (1+r)/(1-r) Degree of Polymerization 1000 100 DP = 1/(1-p) 10 1 0 DP = Degree of polymerization p = mole fraction monomer conversion 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 Mole Fraction Conversion (p) 72 A polyimide is prepared by polycondensation of a diamine with a dianhydride whose repeat unit structure is shown below: O C O C N N C O O C O n Calculate the theoretical molecular weight of the polymer when 183 Kg of diamine (mol wt. 200 g/mol) and 200 Kg of dianhydride (mol wt. 218 g/mol) was used in the reaction, where the extent of reaction is 99%. Calculate the amount of water (in Kg) will produce in this reaction. 73 Nylon-6,6 O O O NaOH Cl 4 Cl Adipoyl chloride H2N 4 NH2 Cl O N H 4 1,6-Diaminohexane O Adipoyl chloride in hexane HO N H 4 O N H 4 4 Nylon 6,6 Diamine, NaOH, in H2O H 6 carbon diacid N H H n 6 carbon diamine Nylon-6,6 74 Nylon-6,6 Since the reactants are in different phases, they can only react at the phase boundary. Once a layer of polymer forms, no more reaction occurs. Removing the polymer allows more reaction to occur. Adipoyl chloride in hexane Nylon 6,6 Diamine, NaOH, in H2O 75 Polyesters, Amides, and Urethanes Monomer Polymer O HO2C CO2H Terephthalic acid OH HO Ethylene glycol NCO 4,4-diisocyantophenylmethane O HO H N H2 H2 O C C O H Poly(ethylene terephthalate n Ester A2 or B2 H2 C OCN HO O H2 C OH HO Spandex Ethylene glycol O H2 H2 H N O C C O H AB HO-R-COOH Urethane linkage n 76 A2 + B2 PET A2 = Terephthalic acid B’2 = Ethylene glycol, HO-CH2-CH2-OH Moles of A2 = Moles of B’2 A2 + B’2 +B’’2 -(PET)-(PBT)- A2 = Terephthalic acid B’2 = Ethylene glycol B’’2= Butylene glycol Moles of A2 = Moles of B’2 +Moles moles of B’’2 HO-CH2-CH2-OH HO-CH2-CH2-CH2-CH2-OH 77 AB Δ Vacuum DL-Lactic acid DLPLA-2/ DLPLA-2 Δ Vacuum DL-Lactic acid Glycolic acid PLGA-7515/ PLGA-7520 78 Physical Properties Linear Polymer Stretch The chains can be stretched, which causes them to flow past each other. When released, the polymer will not return to its original form. Cross-Linked Polymer Stretch Relax The cross-links hold the chains together. When released, the polymer will return to it's original form. 79 Mechanical Properties • Stress and strain: What are they and why are they used instead of load and deformation? • Elastic behavior: When loads are small, how much deformation occurs? What materials deform least? • Plastic behavior: At what point does permanent deformation occur? What materials are most resistant to permanent deformation? • Toughness and ductility: What are they and how do we measure them? 80 Elastic Deformation 1. Initial 2. Small load 3. Unload bonds stretch return to initial d F F Linearelastic Elastic means reversible! d 81 Non-Linearelastic Plastic Deformation (Metals) 1. Initial 2. Small load bonds stretch & planes shear delastic + plastic 3. Unload planes still sheared dplastic F F Plastic means permanent! linear elastic linear elastic dplastic 82 d Engineering Stress • Tensile stress, s: • Shear stress, t: Ft Area, Ao Area, Ao Ft Ft lb f N = 2 or s= 2 in m Ao original area before loading 83 Ft F Fs Fs Fs t= Ao F Stress has units: N/m2 or lbf /in2 Ft Common States of Stress • Simple tension: cable F F A o = cross sectional area (when unloaded) F s s Ao s • Torsion (a form of shear): drive shaft M Ac M 84 Fs Ski lift (photo courtesy P.M. Anderson) Ao Fs t Ao 2R Note: t = M/AcR here. OTHER COMMON STRESS STATES (i) • Simple compression: Ao Canyon Bridge, Los Alamos, NM (photo courtesy P.M. Anderson) Balanced Rock, Arches National Park (photo courtesy P.M. Anderson) 85 F s Ao Note: compressive structure member (s < 0 here). OTHER COMMON STRESS STATES (ii) • Bi-axial tension: Pressurized tank (photo courtesy P.M. Anderson) Fish under water sq > 0 sz > 0 86 • Hydrostatic compression: sh< 0 (photo courtesy P.M. Anderson) Engineering Strain • Tensile strain: • Lateral strain: d/2 e d Lo wo • Shear strain: -dL eL wo Lo dL /2 q g = x/y = tan q x 90º - q y 90º 87 Strain is always dimensionless. Adapted from Fig. 7.1 (a) and (c), Callister & Rethwisch 3e. Stress-Strain Testing • Typical tensile test machine extensometer • Typical tensile specimen specimen Adapted from Fig. 7.2, Callister & Rethwisch 3e. gauge length Adapted from Fig. 7.3, Callister & Rethwisch 3e. (Fig. 7.3 is taken from H.W. Hayden, W.G. Moffatt, and J. Wulff, The Structure and Properties of Materials, Vol. III, Mechanical Behavior, p. 2, John Wiley and Sons, New York, 1965.) 88 Linear Elastic Properties • Modulus of Elasticity, E: (also known as Young's modulus) • Hooke's Law: s=Ee s F E e Linearelastic 89 F simple tension test Poisson's ratio, n • Poisson's ratio, n: eL eL ne metals: n ~ 0.33 ceramics: n ~ 0.25 polymers: n ~ 0.40 Units: E: [GPa] or [psi] n: dimensionless 90 e -n n > 0.50 density increases n < 0.50 density decreases (voids form) Mechanical Properties Slope of stress strain plot (which is proportional to the elastic modulus) depends on bond strength of metal Adapted from Fig. 7.7, Callister & Rethwisch 3e. 91 Other Elastic Properties • Elastic Shear modulus, G: t M G t=Gg • Elastic Bulk modulus, K: V P = -K Vo g M P P K V P Vo • Special relations for isotropic materials: E G 2(1 + n) 92 simple torsion test E K 3(1 - 2n) P pressure test: Init. vol =Vo. Vol chg. = V Young’s Moduli: Comparison Metals Alloys 1200 1000 800 600 400 E(GPa) 200 100 80 60 40 Graphite Composites Ceramics Polymers /fibers Semicond Diamond Tungsten Molybdenum Steel, Ni Tantalum Platinum Cu alloys Zinc, Ti Silver, Gold Aluminum Magnesium, Tin Si carbide Al oxide Si nitride Carbon fibers only CFRE(|| fibers)* <111> Si crystal Aramid fibers only <100> AFRE(|| fibers)* Glass -soda Glass fibers only GFRE(|| fibers)* Concrete 109 Pa 10 8 6 4 2 1 0.8 0.6 0.4 93 GFRE* 20 0.2 CFRE* GFRE( fibers)* Graphite Polyester PET PS PC CFRE( fibers) * AFRE( fibers) * Epoxy only PP HDPE PTFE LDPE Wood( grain) Based on data in Table B.2, Callister & Rethwisch 3e. Composite data based on reinforced epoxy with 60 vol% of aligned carbon (CFRE), aramid (AFRE), or glass (GFRE) fibers. Useful Linear Elastic Relationships • Simple tension: d FL o d -n Fw o L EA o EA o F a wo 2ML o r o4 G M = moment a = angle of twist d/2 Ao dL /2 • Simple torsion: Lo Lo 2ro • Material, geometric, and loading parameters all contribute to deflection. • Larger elastic moduli minimize elastic deflection. 94 Plastic (Permanent) Deformation (at lower temperatures, i.e. T < Tmelt/3) • Simple tension test: Elastic+Plastic at larger stress engineering stress, s Elastic initially permanent (plastic) after load is removed ep engineering strain, e plastic strain 95 Adapted from Fig. 7.10 (a), Callister & Rethwisch 3e. Yield Strength, sy • Stress at which noticeable plastic deformation has occurred. when ep = 0.002 tensile stress, s sy sy = yield strength Note: for 2 inch sample e = 0.002 = z/z z = 0.004 in engineering strain, e ep = 0.002 96 Adapted from Fig. 7.10 (a), Callister & Rethwisch 3e. Yield Strength : Comparison Metals/ Alloys 2000 Graphite/ Ceramics/ Semicond Polymers Composites/ fibers 97 200 Al (6061) ag Steel (1020) hr Ti (pure) a Ta (pure) Cu (71500) hr 100 70 60 50 40 Al (6061) a 30 20 10 Tin (pure) ¨ dry PC Nylon 6,6 PET PVC humid PP HDPE LDPE Hard to measure, 300 in ceramic matrix and epoxy matrix composites, since in tension, fracture usually occurs before yield. 700 600 500 400 Ti (5Al-2.5Sn) a W (pure) Cu (71500) cw Mo (pure) Steel (4140) a Steel (1020) cd since in tension, fracture usually occurs before yield. 1000 Hard to measure , Yield strength, sy (MPa) Steel (4140) qt Room temperature values Based on data in Table B.4, Callister & Rethwisch 3e. a = annealed hr = hot rolled ag = aged cd = cold drawn cw = cold worked qt = quenched & tempered Tensile Strength, TS • Maximum stress on engineering stress-strain curve. Adapted from Fig. 7.11, Callister & Rethwisch 3e. TS F = fracture or ultimate strength engineering stress sy Typical response of a metal Neck – acts as stress concentrator strain engineering strain • Metals: occurs when noticeable necking starts. • Polymers: occurs when polymer backbone chains are aligned and about to break. 98 Tensile Strength: Comparison Metals/ Alloys Tensile strength, TS (MPa) 5000 3000 2000 1000 300 200 100 40 30 Polymers 20 Composites/ fibers C fibers Aramid fib E-glass fib Steel (4140) qt W (pure) Ti (5Al-2.5Sn)aa Steel (4140)cw Cu (71500) Cu (71500) hr Steel (1020) Al (6061) ag Ti (pure) a Ta (pure) Al (6061) a AFRE(|| fiber) GFRE(|| fiber) CFRE(|| fiber) Diamond Si nitride Al oxide Si crystal <100> Glass-soda Concrete Nylon 6,6 PC PET PVC PP HDPE Graphite wood(|| fiber) GFRE( fiber) CFRE( fiber) AFRE( fiber) LDPE 10 wood ( 1 99 Graphite/ Ceramics/ Semicond fiber) Room temperature values Based on data in Table B4, Callister & Rethwisch 3e. a = annealed hr = hot rolled ag = aged cd = cold drawn cw = cold worked qt = quenched & tempered AFRE, GFRE, & CFRE = aramid, glass, & carbon fiber-reinforced epoxy composites, with 60 vol% fibers. Ductility • Plastic tensile strain at failure: Lf - Lo x 100 %EL Lo smaller %EL Engineering tensile stress, s larger %EL Lo Ao Af Adapted from Fig. 7.13, Callister & Rethwisch 3e. Engineering tensile strain, e • Another ductility measure: 100 %RA = Ao - Af x 100 Ao Lf Toughness • Energy to break a unit volume of material • Approximate by the area under the stress-strain curve. Engineering tensile stress, s small toughness (ceramics) large toughness (metals) very small toughness (unreinforced polymers) Adapted from Fig. 7.13, Callister & Rethwisch 3e. Engineering tensile strain, e Brittle fracture: elastic energy Ductile fracture: elastic + plastic energy 101 Resilience, Ur Ability of a material to store energy Energy stored best in elastic region Ur ey 0 sde If we assume a linear stress-strain curve this simplifies to 1 Ur @ sy e y 2 Adapted from Fig. 7.15, Callister & Rethwisch 3e. 102 Elastic Strain Recovery sy i D syo Stress 2. Unload 1. Load 3. Reapply load Strain Adapted from Fig. 7.17, Callister & Rethwisch 3e. 103 Elastic strain recovery Mechanical Properties Ceramic materials are more brittle than metals. Why is this so? Consider mechanism of deformation In crystalline, by dislocation motion In highly ionic solids, dislocation motion is difficult few slip systems resistance to motion of ions of like charge (e.g., anions) past one another 104 Flexural Tests – Measurement of Elastic Modulus • Room T behavior is usually elastic, with brittle failure. • 3-Point Bend Testing often used. -- tensile tests are difficult for brittle materials. F cross section L/2 d b rect. L/2 Adapted from Fig. 7.18, Callister & Rethwisch 3e. R d = midpoint circ. deflection • Determine elastic modulus according to: F x slope = F d d linear-elastic behavior 105 F L3 E d 4bd 3 (rect. cross section) F L3 (circ. cross section) E 4 d 12R Flexural Tests – Measurement of Flexural Strength • 3-point bend test to measure room-T flexural strength. cross section d b rect. L/2 F L/2 Adapted from Fig. 7.18, Callister & Rethwisch 3e. R d = midpoint circ. deflection location of max tension • Flexural strength: sfs sfs 106 3Ff L 2bd 2 Ff L R 3 • Typical values: sfs (MPa) E(GPa) Si nitride 250-1000 304 Si carbide 100-820 345 Al oxide 275-700 393 glass (soda-lime) 69 69 Material (rect. cross section) (circ. cross section) Data from Table 7.2, Callister & Rethwisch 3e. Mechanical Properties of Polymers – Stress-Strain Behavior brittle polymer plastic elastomer elastic moduli – less than for metals Adapted from Fig. 7.22, Callister & Rethwisch 3e. • Fracture strengths of polymers ~ 10% of those for metals • Deformation strains for polymers > 1000% 107 – for most metals, deformation strains < 10% 107 Influence of T and Strain Rate on Thermoplastics • Decreasing T... -- increases E -- increases TS -- decreases %EL • Increasing strain rate... -- same effects as decreasing T. s(MPa) 80 4°C 60 20°C 40 Plots for semicrystalline PMMA (Plexiglas) 40°C 20 0 60°C 0 0.1 0.2 e to 1.3 0.3 Adapted from Fig. 7.24, Callister & Rethwisch 3e. (Fig. 7.24 is from T.S. Carswell and J.K. Nason, 'Effect of Environmental Conditions on the Mechanical Properties of Organic Plastics", Symposium on Plastics, American Society for Testing and Materials, Philadelphia, PA, 1944.) 108 108 Time-Dependent Deformation • Stress relaxation test: -- strain in tension to eo and hold. -- observe decrease in stress with time. tensile test eo s(t) time • Relaxation modulus: 109 for T > Tg. 5 10 Er (10 s) 3 in MPa 10 rigid solid (small relax) transition region 1 10 10-1 strain s(t ) E r (t ) eo • There is a large decrease in Er viscous liquid 10-3 (large relax) (amorphous polystyrene) Adapted from Fig. 7.28, Callister & Rethwisch 3e. (Fig. 7.28 is from A.V. Tobolsky, Properties and Structures of Polymers, John Wiley and Sons, Inc., 1960.) 60 100 140 180 T(°C) Tg • Representative Tg values (C): PE (low density) PE (high density) PVC PS PC - 110 - 90 + 87 +100 +150 Selected values from Table 11.3, Callister & Rethwisch 3e. 109 Hardness • Resistance to permanently indenting the surface. • Large hardness means: -- resistance to plastic deformation or cracking in compression. -- better wear properties. apply known force measure size of indent after removing load e.g., 10 mm sphere D most plastics brasses Al alloys Smaller indents mean larger hardness. d easy to machine steels file hard cutting tools increasing hardness 110 nitrided steels diamond Hardness: Measurement Rockwell No major sample damage Each scale runs to 130 but only useful in range 20100. Minor load 10 kg Major load 60 (A), 100 (B) & 150 (C) kg A = diamond, B = 1/16 in. ball, C = diamond HB = Brinell Hardness TS (psia) = 500 x HB TS (MPa) = 3.45 x HB 111 Hardness: Measurement 112 True Stress & Strain Note: S.A. changes when sample stretched True stress sT s1 + e sT F Ai True strain eT ln i o eT ln1 + e Adapted from Fig. 7.16, Callister & Rethwisch 3e. 113 Hardening s • An increase in sy due to plastic deformation. large hardening sy 1 sy small hardening 0 e • Curve fit to the stress-strain response: sT K eT “true” stress (F/A) 114 n hardening exponent: n = 0.15 (some steels) to n = 0.5 (some coppers) “true” strain: ln(L/Lo) Variability in Material Properties Elastic modulus is material property Critical properties depend largely on sample flaws (defects, etc.). Large sample to sample variability. Statistics n Mean xn x n 1 2 2 Standard Deviation n x i - x s n -1 where n is the number of data points 115 Design or Safety Factors • Design uncertainties mean we do not push the limit. • Factor of safety, N Often N is sworking sy between 1.2 and 4 N • Example: Calculate a diameter, d, to ensure that yield does not occur in the 1045 carbon steel rod below. Use a factor of safety of 5. sworking 220,000N d /4 2 5 sy N 1045 plain carbon steel: sy = 310 MPa TS = 565 MPa d = 0.067 m = 6.7 cm 116 F = 220,000N d Lo • Stress and strain: These are size-independent measures of load and displacement, respectively. • Elastic behavior: This reversible behavior often shows a linear relation between stress and strain. To minimize deformation, select a material with a large elastic modulus (E or G). • Plastic behavior: This permanent deformation behavior occurs when the tensile (or compressive) uniaxial stress reaches sy. • Toughness: The energy needed to break a unit volume of material. • Ductility: The plastic strain at failure. 117 Tensile strength function of Mol. Wt. of the polymer Strength as a function of molecular weight. At low molecular weight, the strength is too low for the polymer material to be useful. At high molecular weight, the strength increases eventually saturating to the infinite molecular weight result of S∞ . 118 Mechanical Properties i.e. stress-strain behavior of polymers brittle polymer sFS of polymer ca. 10% that of metals plastic elastomer elastic modulus – less than metal Strains – deformations > 1000% possible (for metals, maximum strain ca. 10% or less) 119 Melting and Glass Transition Temperature • Melting of a crystalline polymer – transforming solid with an ordered structure to a viscous liquid with a highly random structure • Amorphous glass transitions – transformation from a rigid material to one that has rubberlike characteristics – temperature has large effect on chain flexibility • Below glass transition temperature, Tg, polymers are usually brittle and glass-like in mechanical behavior. • Above glass transition, Tg, polymers are usually more elastic. Why is That? Bond rotations are “freezing” which means chains can’t slip past each other so polymer becomes brittle, (no plastic deformation) 120 Melting vs. Glass Transition Temp. What factors affect Tm and Tg? Both Tm and Tg increase with increasing chain stiffness Chain stiffness increased by 1. Bulky side groups 2. Polar groups or side groups 3. Double bonds or aromatic chain groups Regularity – effects Tm only 121 121 DSC plot of semicrystalline polymers 122 Thermal Analysis Differential Scanning Calorimetry (DSC) Measure heat absorbed or liberated during heating or cooling Thermal Gravimetric Analysis (TGA) Measure change in weight during heating or cooling Differential Scanning Calorimetry (DSC) Calorimeter-Heat flow in sample Differential calorimeter-heat flow in sample vs referance as function of time (mJ/sec). •Differential Scanning Calorimetry (DSC) measures the temperatures and heat flows associated with transitions in materials as a function of time and temperature in a controlled atmosphere. •These measurements provide quantitative and qualitative information about physical and chemical changes that involve endothermic or exothermic processes, or changes in heat capacity. Conventional DSC Empty Sample Metal 1 Sample Temperature Metal Metal 2 1 Metal 2 Reference Temperature Temperature Difference = Heat Flow •A “linear” heating profile even for isothermal methods Modes and principles of operation (1) (b) Power compensated DSC: Temperature differences between the sample and reference are ‘compensated’ for by varying the heat required to keep both pans at the same temperature. The energy difference is plotted as a function of sample temperature (c) Heat flux DSC ultilizes a single furnace. Heat flow into both sample and reference material via an electrically heated constantan thermoelectric disk and is proportional to the difference in output of the two themocouple junctions a) DTA: difference in temperature between the sample and reference is plotted against sample temperature Diagram of a DSC apparatus • A DSC apparatus is built around - a differential detector - a signal amplifier - a furnace - a temperature controller - a gas control device - a data acquisition device Gas control Sample Furnace Reference Detectors Microvolt amplifier Furnace controller Data acquisitio n DSC measures:•Glass transitions •Melting and boiling points •Crystallisation time and temperature •Percent crystallinity •Heats of fusion and reactions •Specific heat capacity •Oxidative/thermal stability •Rate and degree of cure •Reaction kinetics •Purity Heat Flow -> exothermic DSC Thermogram Cross-Linking (Cure) Crystallisation Glass Transition Melting Temperature 6 Oxidation Main Sources of Errors •Calibration •Contamination •Sample preparation – how sample is loaded into a pan •Residual solvents and moisture. •Thermal lag •Heating/Cooling rates •Sample mass •Processing errors Sample preparation Form of sample: bulk solid, powder (pressed), liquid. Amount of sample: 3-5mg. DSC Pan: Al, Pt, stainless steel, Ag, Cu, Al2O3 Sample Preparation : Shape • Keep sample as thin as possible (to minimise thermal gradients) • Cover as much of the pan bottom as possible • Samples should be cut rather than crushed to obtain a thin sample (better and more uniform thermal contact with pan) 99 Operation procedures Calibration of instrument •Temperature, heat of reaction, heat capacity scale using high purity standards (In, Sn, Bi, Pb,Au) •Baseline correction for a given scan rate (1 40K/min). •Weight samples before (and maybe after) experiment. Type of DSC experiments 1. 2. Dynamic heating - thermodynamic properties Isothermal heating - kinetic parameters 1. Dynamic heating:- Constant heat rate mode. (e.g. heat flow vs. temperature). What can we characterise? Information: 1. Transformation temperature (e.g. onset, peak). 2. Transformation enthalpy (e.g. area under the peak). 3. Activation energy for transformation (e.g. Kissinger analysis) 2.)Isothermal heating mode:Heating at a fixed temperature over a time interval.(e.g. heat flow vs. time) THERMOGAVIMETRIC ANALYSIS (TGA) Principle: TGA measures the amount and the rate of weight change of a material with respect to temperature or time in controlled environments. . A TGA consists of three major parts a furnace, 1. A microgram balance, 2. An auto sampler and 3. A thermocouple. Instrument Instrument used for thermogravimetry is “Thermobalance”. Data recorded in form of curve known as ‘Thermogram’ The furnace can raise the temperature as high as 1000°C or more which is made of quartz. The auto sampler helps to load the samples on to the microbalance. The thermocouple sits right above the sample. Care should be taken at all times that the thermocouple is not in touch with the sample which is in a platinum pan. • A technique that permits the continuous weighing of a sample as a function of temperature and/or as a function of time at a desired temperature TGA Curve of Calcium Oxalate 142 143 TGA plot of (a) PS, (b) Pure PC, (c) PC/Carbonyl Iron Composite 145 146 147 10 oC/min, air 148 149 Sample Preparation Sample preparation has a significant effect in obtaining good data. It is suggested that maximizing the surface area of the sample in a TGA pan improves resolution and reproducibility of weight loss temperatures. The sample weight affects the accuracy of weight loss measurements. Typically 10-20mg of sample is preferred in most applications. Whereas, if the sample has volatiles 50-100mg of sample is considered adequate. It is to be noted that most TGA instruments have baseline drift of ±0.025mg which is ±0.25% of a 10mg sample. Experimental Conditions Heating Rate Purge gas Experimental Conditions -Heating Rate Samples are heated at a rate of 10 or 20°C/min in most cases. Lowering the heating rates is known to improve the resolution of overlapping weight losses. Experimental Conditions -Purge gas • Nitrogen is the most common gas used to purge samples in TGA due to its inert nature. • Whereas, helium provides the best baseline. • Air is known to improve resolution because of a difference in the oxidative stability of components in the sample. • Vacuum may be used where the sample contains volatile components, which helps improve separation from the onset of decomposition since the volatiles come off at lower temperatures in vacuum. • e.g. oil in a rubber tire product. Calibration Blank test Calibration of mass changes Calibration of temperature Applications of TGA Determination of the bound and unbound water in the suspension of Milk of Magnesia (MoM), used as a laxative. In an overview of thermal analysis testing it is always preferable to do a TGA experiment on unknown samples before doing a DSC experiment (especially for pharmaceuticals). Decomposition of pharmaceuticals renders products which are insoluble and generally sticky on the inside of a DSC cell. These products will lower the life use of a DSC cell. Therefore, know the decomposition temperatures of all drugs and heat in a DSC evaluation to 50°C below those temperatures. Applications of TGA Evaporation of free (unbound) water begins at room temperature due to dry gas flowing over the sample. Dehydration/Desolvation of bound water almost always begins at temperatures above room temperature and typically 125°C. Decomposition can have multiple stages (weight losses) but the presence of multiple weight loss steps can also indicate the presence of multiple components in the sample. Tg of PMMA at different heating rate 155 Tg and Tm 156 10 oC/min 100 95 300 oC Wt % 0 Time / temp 200 h 157 Modulus of different calss of polymers w.r.t. temperature 158 Applications of Polymers It is difficult to find an aspect of our lives that is not affected by polymers. Just 70 years ago, materials we now take for granted were non-existent. 1. 2. 3. 4. 5. 6. 7. 8. 9. Polymers in daily life: Polymers in automotive Polymers in sports Polymers in agricultural Polymers in medicine Polymers pharmacy and pharmaceutical formulations Polymers in aero-space Polymers in electronics Polymers in defence 159 Electronic Packaging 160 The need for low dielectric constant materials • Smaller feature size in microelectronic devices leads to increasing line to line capacitance t = RC = 2 e e0 [4L2 / P2 + L2 / T2] R C e e0 L, P, T 161 = Total resistance = Total Capacitance = Specific resistance of the metal of the interconnect lines. = Dielectric constant of the insulator between the Inter connect lines. = Dielectric constants of vacuum. = Length, pitch & thickness of the interconnect array. Signal delay vs. device size in integrated circuitry Ref: S.-P. Jeng et al. Mat. Res. Soc. Symp. Proc. 337, 25 (1994)]. 162 SEMATECH roadmap for intermetal dielectric Required dielectric constant Technology year 1998 2001 2004 2007 Design rule (micron) 0.25 0.18 0.15 0.12 Required e 3.9 2.9 2.3 1.9 Ref: Semiconductor Industry Association. International Technology Roadmap for semiconductors (ITRS), 1998 update (http://www.itrs.net/ntrs/publntrs.nsf) 163 Book: Properties of Polymers, Fourth Edition Their Correlation with Chemical Structure their Numerical Estimation and Prediction from Additive Group Contributions Authors: D.W. van Krevelen, Klaas te Nijenhuis O SiO2 e = 4 164 KAPTON O O N N O O e = 3.4 Tg > 300 0C TS = 158 MPa YM = 3.2 GPa Water Absorption = 3.5 % n Properties required for new intermetal Dielectrics • Dielectric constant < 3, preferably 2.5 • Dissipation factor at 1 MHz < 0.005 • Thermal stability 400 - 450 0C • Glass transition temperature 300 0C • Thermal conductivity <0.5 W/(m.K) • Coefficient thermal expansion < 50 ppm • Tensile strength >100 MPa • Young modulus >1 GPa • Moisture absorption < 1% • Good adhesion to materials such as SiO2, Si, Al, Cu, TiN • Good film forming ability Ref: P. Springer, Semiconductor. Int. 5 (1996) 88 165 Solutions Spin-on Plasma polymerization • Well defined chemical structure • Good local planarization • Post deposition cure • Solvent free • Good uniformity • Common equipment can be used Concept For low dielectric constant: • Absence of polar groups • Incorporation of fluorine as pendent CF3 group For high Tg and strength: • Stiff repeat unit • High molecular weight 166 Spin-on FLARE F F F F O Ar O n F F F e Tg Td TS YM CTE Gap Fill Property 167 F = 2.5-3.1 = 189-285 oC = 540 oC = 83-107 MPa = 2.45-2.95 GPa = 65- 76 ppm / oC = < 0.8 m SiLK Plasma polymerization o X 400-450 C X O X = S, O, H3C CH3 F3C C 168 C , C CF3 e = 2.6 2.7 X Plasma polymerization Crosslinked BCB resins OCH3 OCH3 Si Si O OCH3 OCH3 T OCH3 OCH3 Si Si O OCH3 169 OCH3 e TS YM CTE Water absorption = 2.6-2.7 = 85 MPa = 2 GPa = 52 ppm / oC = 0.2 % Poly(perfluorocyclobutane) (PFCB) F F F CH3 O F C F O F O F Heat F F FF F CH3 F O C FF F O FF CH3 F O C FO FO F F F F FF F F FO FF F F O C CH3 FF FO F F F F F 170 F O F FF FF F FF e = 2.35 Tg = 400 oC TS = 66 MPa YM = 2.27 GPa EB =4% Poor Thermal Stability Plasma polymerization Parylene F F2C F2C e Tg Td YM CTE 171 CF2 600 oC 2 F2C CF2 F2C CF2 CF2 = 2.2-2.4 = 160 0C = 530 0C = 2.41 GPa = 28 ppm / 0C Designing new polymers by molecular engineering F3 C Polyamide/Polyimide H 2N O Ar O NH2 CF3 F3 C CF3 F F hbp F CF3 Ar CF3 F3C F F3 C Br CF3 F3 C F F3 C F F3 C F HO Polyether hbp F B(OH)2 F H 3C F CF3 OH F3C Polyether F F F O2N H 3C CF3 F3 C O2N O Y O BrH2C CH2Br O O NO2 CF3 PPV derivative F3 C H 2N O Y O NH2 Polyamide/Polyimide CF3 Courtesy of 5-bromo-2-fluoro benzotrifluoride Spin-on Poly(arylene ether) CF3 O O n F3C e = 2.67; Td = 534 0C (5 % wt. loss in air) Tg = 300 / 315 0C (DSC / DMTA) TS = 75 MPa Water absorption rate = 0.2 wt % Ref : S. Banerjee, et al. Chem. Mater (1999) 173 Spin-on Poly(ether imide) e = 2.60; Water abs. = 0.2 wt %; Td = 526 0C; Tg = 273 0C; Ts = 109 MPa ; Mod.= 2.5 GPa; Eb = 24 % Ref. S. Banerjee, et al. J. Polym. Sci. Polym. Chem. (2002) 174 n Summary of the polymer properties O N O Ar Q CH3 Q O N CH3 CH3 O F3C CH3 O CH3 CF3 CF3 F3 C F3C CF3 O CF3 262 12.94 3.12 0.45 252 20.59 2.79 0.28 239 11.57 3.09 0.48 230 11.72 3.05 0.53 268 23.05 2.92 0.21 260 30.56 2.52 0.18 245 20.86 2.62 0.28 235 21.09 2.57 0.26 311 11.36 2.81 0.48 285 18.55 2.58 0.38 282 10.29 2.92 0.51 275 10.41 2.90 0.47 CF3 CF3 O Ar % Water abs. O CH3 CF3 % Dielectric Fluorine constant CF3 CH3 CH3 Tg / 0C O CF3 CF3 O CF3 F3C CF3 O O Hyperbranched polymers B A B Schematic representation of a hyperbranched polymer by AB2 monomer [DBFréchet = (t+d)/(t+l+d)] B + A A DB for linear polymer = 0 DB for hyperbranched polymer = 0-1 B A B B B B A A + B A A B B [DBFrey = 2d/(2d+l)] DB for perfect dendrimer = 1 B B A B B B B + A B' A B A A B' B' B' Schematic routes for hyperbranched polymers Hyperbranched polymer Concept • Higher fractional free volume • Higher fluorine content 11' F3 C 8' 7' 6' 9 F 10 F3 C 3 5 5' 10' 8 4 6 NMP / toluene O K2CO3 / 180°C CF3 7 11 2 4' 1' 2' 3' 1 5 10 6 OH AB2 9' F 9 7 8 F3 C 11 + F hb,n hbp(lw) & hbp(hw) R HO R 11' OH F3 C R O 8' 12 7' A2 6' 15 17 5' 10' R = CH3, CF3 NMP / toluene O 4' 1' K2CO3 / 180°C R= 2' 3' CH3 F3 C 11 Normalized heat flow (W/g) endo up O 16 R 13 14 9' C hbp(lw) hbp(hw) 2a 2a 3a 1:1 3:2 2b 3b 3:2 7:6 2c 3c 2:1 4:4 100 2b 3a AB2 / A2 OH / F CF3 75 Weight % 3b 2c 3c Td10% = 573 0C Tg > 350 0C Mw = 44,70,000 gmol-1 PDI = 2.2 2c hbp(hw) 3c 50 25 0 50 100 150 200 250 o Temperature C 300 350 0 100 200 300 400 500 600 700 800 O Temperature C A. Ghosh et.al. Macromolecules 2010 Energy Saving 178 Membrane Based Separation Processes Process Pore size/ mol wt Driving Force Mechanism Advantages Microfiltration 0.02-10μm ΔP 0-1 bar Sieving • Low energy consumption. Ultrafiltration 0.001-0.02μm mol wt 103-106 ΔP 0-10 bar Sieving • Low investment cost. Nanofiltration <2 nm mol wt 102-103 ΔP 10-25 bar Solution Diffusion Reverse Osmosis non porous ΔP 10-100 bar Solution Diffusion Gas Separation non porous ΔP 10-100 bar Solution Diffusion Dialysis 10-30 A Concentration difference Sieving plus Diffusion Pervaporation non porous Partial pressure difference Solution Diffusion Electrical potential Ion Migration Electrodialysis mol wt <200 • Better selectivity without thermodynamic limitations. • Clean and close operation. • No process wastes. • Compact and scalable units. Drawbacks • Scarce membrane market. • Lack of information. • Low permeate flows. • Limited application 1. Sorption Organic substances dehydration 2. Diffusion 3. Desorption Recovery of volatile compounds at low concentrations Separation of close boiling as well as azeotropic mixtures Polyimides Kapton® Strengths High thermal stability High glass transition temperatures (above 350 oC) High mechanical properties Good chemical resistance Low dielectric constant Ref. H. Ohya, V.V. Kudryavtsev, S.I. Semenova, Polyimide membranes: Applications, fabrications, and properties. Gordon and Breach, Tokyo, 1996. Industrial Applications Insulating films for flexible cables in electrical industry High temperature adhesive in semiconductor industry High temperature resistive cables for aerospace application Machinery parts and shapes for automobile application Photoresist material in photolithography Membrane based gas separation application Ref. N.L. Norman, G.F. Anthony, W.S. Winston Ho, Advanced membrane technology and applications. John Wiley & Sons Inc; 2008. Scope of gas separation process Recovery of excessive flue gases from heavy industries effluents. Removal of CO2, H2S and H2O from natural gas to increase the fuel efficiency and decrease pipe corrosion. Preparation of oxygen enriched air. Other technologies Amine adsorption process Cryogenic distillation process Gas Separation Membrane Market Value, 2000 Total Value : $127 mililion Ref. F.P. McCandless, Ind. Eng. Chem. Process Des. Dev. 1972, 11, 470. W.J. Koros, G. K. Fleming, J. Membr. Sci. 1993, 83, 1. Commercially Used Polymers for Gas Separation Permeability (Barrers) Polymer O O Ideal permselectivity α (CO2/CH4) Ideal permselectivity α (O2/N2) CO2 O2 9.0 1.90 37.5 7.0 1.33 0.41 36.9 8.0 3.28 0.81 25.2 6.2 5.6 1.4 22.4 5.6 6.56 1.46 32.8 6.4 O N N O n O Matrimid O O O O N N O n O Ultem O O O O N O S O N O O n Extem O O O S O Polysulfone H3COCOH2C O O OH n H3COCO Cellulose acetate n Ref. T.S. Chung et al., Ind. Eng. Chem. Res. 10.1021/ie901906p A.C. Puleo, D.R. Paul, Journal of Membrane Science, 47 (1989) 301. Design concept Solution diffusion mechanism Fixed O N O G. Maier, Angew. Chem. Int. Ed. 1998, 37, 2960. O Ar' N O O F3C Ar CF3 O O N O O Ar' N O O O N O Matrimid® 5218 Resin O Scope of membrane research N O n CO2 recovery from land fill gas CO2 ~ 40-45 mol % CH4 ~ 54 – 59 mol % N2 ~ 4 %, O2 ~ 1 % Water vapor ~ 1 % H2S & CFC ~ trace Capture of CO2 from the flue gas (CO2 ~ 15 %, water ~ 7 %, O2 ~ 3 %, Nitrogen ~74 %, NOx + SOx ~ 1 %) Commercial membranes are limited to ambient temperature Matrimid offers high temperature separation of CO2 Mixed matrix membranes CMS dispersed within polymer matrices showed manifold increase in CO2 permeability and CO2 / CH4 selectivity Present need: Membrane that can operate at high temperature and pressure Higher flux and selectivity Low cost (for large scale application) Measurement of Gas Transport Properties CO2 CH4 Figure: Schematic diagramme of the instrumental setup P = Permeability coefficient V = downstream volume (119 cm3) D = Diffusion coefficient (cm2/sec) A = area of membrane (5.069 cm2 ) O2 pi = upstream gas pressure (3.5 atm.) αA/B = Ideal permselectivity towards gas ‘A’ relative to gas ‘B’ T0 = 273 K , p0 = 1atm. T = experimental temperature d = thickness of membrane 1 Barrer = 10 -10 cm3 (STP) cm/cm2 s cm Hg N2 Calculation of Gas Transport Coefficients (dp/dt)s = downstream pressure increment per unit time at steady state θ = time-lag dp dt θ Where we stand? Membrane type Permeability (barrers) Structure O CF3 BAPY-6FDA O O N F3 C N CF3 1.95 26.6 51.91 2.11 24.6 52.98 1.21 43.8 53.85 1.01 53.3 O O F3 C CH3 O O F3 C O CF3 N N n O CH3 H 3C SBPDA-6FDA 51.92 n O H3C CH4 O CF3 N F3 C BPI-6FDA CO2 Ideal selectivity P(CO2)/P(CH4) CH3 O F3 C O F3 C O CF3 O O N N n F3 C H 3C O CH3 O O N CF3 BAPA-6FDA O O O F3 C O CF3 N N n O F3 C O CF3 BIDA-6FDA O O N O F3C CF3 O N n O N O F3 C O O 71.32 1.99 35.8 S. K. Sen, Banerjee et al., J. Membr. Sci. Vol. Vol. 343, pages 97-103(2009) S. K. Sen, Banerjee et al., J. Membr. Sci. Vol. 350, pages 53-61 (2010) B. Dasgupta , Banerjee et al., J. Membr. Sci. Vol. 345, pages 249-256 (2009) A. Bos et al., Sep Purif Technol. 1998, 14, 27; W. J. Koros et al., J. Membr. Sci. 2008, 175, 181; and H. Yamamoto et al., J. Polym. Sci. Part B: Polym. Phys. 1990, 28, 2291. P(CO2)/P(CH4) Comparison of CO2/CH4 Separation Performances with Commercially Used Polymers 1000 Matrimid 100 10 0.1 Ultem Extem Polysulfone Cellulose acetate BAPA-PMDA BAPA-6FDA BAPA-BTDA BAPA-ODPA BAPA-BPADA SBPDA-BPADA SBPDA-6FDA SBPDA-ODPA BAQP-6FDA BATP-6FDA BAPy-6FDA BATh-6FDA BIDA-BPADA BIDA-6FDA BIDA-BTDA BIDA-ODPA 1 Present Upper Bound 10 P(CO2) in Barrers 100 Comparison of O2/N2 Separation Performances with Commercially Used Polymers P(O2)/P(N2) 100 10 1 0.01 Matrimid Ultem Extem Polysulfone Cellulose acetate BAPA-PMDA BAPA-6FDA BAPA-BTDA BAPA-ODPA BAPA-BPADA SBPDA-BPADA SBPDA-6FDA SBPDA-ODPA BAQP-6FDA BATP-6FDA BAPy-6FDA BATh-6FDA BIDA-BPADA BIDA-6FDA BIDA-BTDA BIDA-ODPA 0.1 Present Upper Bound 1 P (O2) in Barrers 10 100 Sulzer Chemtech AG, P.O. Box 65 CH-8404, Winterthur, Switzerland BORSIG Membrane Technology GmbH Egellsstraße 21, 13507 Berlin, Germany Betheniville, France H Pervaporation H Why Benzene/Cyclohexane? H H Industrial demand Close boiling mixtures Form azeotropes Easily detected by GC H H H H H H H bp (0C): 80.100 fp (0C): 5.533 H H H H H H H bp (0C): 80.738 fp (0C): 6.554 Bz + Cy (45 wt%): bp 77.5 (0C) Different polymers used for Benzene/Cyclohexane Membrane Benzene in feed Temperature (0C) Normalized Flux (J) (kg μm/m2h) Separation factor (α) Reference LDPE 50 25 10.8 1.6 Huang et al 1968 PVDF 53 56 1.5 5.4 McCandless et al 1974 PVA/PAAm 50 25 2.3 11.9 Park et al 1994 Modified nylon 6 50 50 0.057 4.5 Yoshikawa et al 1997 PAS 50 50 2.6 22.5 Ray et al 1997 PU-TEOS 50 50 0.65 19 Kusakabe et al 1998 BAPPP-TIPA 50 50 16.3 4.9 Wang et al 2001 BAPPH-TIPA 50 50 29.4 4 Wang et al 2001 LDPE: Low density polyethylene; PVDF: Poly(vinylidene fluoride); PVA/PPAm: Polyvinyl alcohal/ Polyallyl amine; PAS: Poly(acrylonitrile-co-styrene); PU-TEOS: Polyurethane-tetraethyl-ortho-silicate); BAPPP: 2,2-Bis[4-(4-amionphenoxy) phenyl]propane; BAPPH: 2,2-Bis[4-(4-amionphenoxy)phenyl]hexafluoro propane; TIPA: 5-t-butyl-isophthalic acid. H Why Benzene/Cyclohexane? Industrial demand Close boiling mixtures Form azeotropes Easily detected by GC H H H H H H bp (0C): 80.100 fp (0C): 5.533 Benzene H H H H H H H H H bp (0C): 80.738 fp (0C): 6.554 Bz + Cy (45 wt%): bp 77.5 (0C) Ethyl benzene Cumene Cyclohexane Aniline Chlorobenzenes Styrene Adipic acid Caprolactam Polystyrene Nylon 6,6 Nylon 6 Polycarbonate Bisphenol A Acetone H Epoxy resins Phenol Phenolic resin H Goal of the present research Design and preparation of new diamine monomers Preparation of processable PEAs Systematic investigation of pervaporation study of the PEAs for benzene (Bz)/cyclohexane (Chx) mixture at different temperatures (50, 60 and 70 oC) Establishment of structure property correlationship Pervaporation application 10% OV-17 packed column Flame ionization detector (FID) Temperature of the oven, injector and detector 80, 100 and 200 oC Chx Bz Bz Chx Polymer Structure vs. Properties PEAs X FFV Separation factor (α) Normalized flux (kg.μm/m2.h) at 50 oC 250 0.188 4.7 23.66 2087 252 0.287 4.4 29.89 2421 273 0.293 3.9 31.42 2169 310 0.254 5.9 30.85 3601 233 0.143 6.9 13.41 1888 240 0.176 6.5 21.77 2849 294 0.173 5.9 23.91 2788 300 0.145 7.6 23.03 3616 230 0.167 6.2 18.04 2236 233 0.201 5.7 24.86 2782 285 0.199 5.0 27.31 2600 290 0.168 7.1 26.04 3782 Ar -(CH3)2120 o Tg (oC) -(CF3)2- (a) a PSI (g/m2.h) at 50 oC O N -(CH3)2180 o -(CF3)2- (b) 120 o b -(CH3)2-(CF3)2- (c) c O N 100 Pervaporation performances in comparison with commercially used polymers Separation factor (a) O 10 1 0.01 PVA; 27 C O PUU; 25 C O PEA; 50 C O CD/PU; 25 C O PEA-Ia; 50 C O PEA-IIa; 50 C O PEA-IIIa; 50 C O PEA-IVa; 50 C O PEA-Ib; 50 C O PEA-IIb; 50 C O PEA-IIIb; 50 C O PEA-IVB; 50 C O PEA-Ic; 50 C O PEA-IIc; 50 C O PEA-IIIc; 50 C O PEA-IVc; 50 C 0.1 Upper bound trade-off curve Lue and Peng, 2003 1 10 100 2 Normalized flux (kg.m/m .h) 1000 Renewable Energies 198 Conjugated polymers Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity 1. Conjugated organic polymers can be doped, via oxidation or reduction chemistry or via acidbase chemistry, to induce very high electrical conductivity. 2. Conjugated polymers are beginning to fnd uses, in both the neutral and the doped states, in prototype molecular-based electronics applications and in electronic and opto-electronic devices. 199 Two conditions to become conductive: 1. The first condition for this is that the polymer consists of alternating single and double bonds, called conjugated double bonds. In conjugation, the bonds between the carbon atoms are alternately single and double. Every bond contains a localised “sigma” (σ) bond which forms a strong chemical bond. In addition, every double bond also contains a less strongly localised “pi” (π) bond which is weaker. 2. The second condition is that the plastic has to be disturbed - either by removing electrons from (oxidation), or inserting them into (reduction), the material. The process is known as doping. 200 Discovery of conducting polymers 1862 Lethby (College of London Hospital) Oxidation of aniline in sulfuric acid 1970’s Shirakawa (Japan) Acetylene gas CH Ti(OBu)4 & Et3Al Toluene Ti(OBu)4 & Et3Al Hexadecane –78oC 150oC copper-coloured film cis-polyacetylene 201 HC silvery film trans-polyacetylene Discoverers - Nobel Prize 2000 A. Heeger, A. McDiarmid, H. Shirakawa 202 Polyacetylene (PA) n n Electrical conductivity (s) cis PA trans PA 10-10 – 10-9 S cm-1 10-5 – 10-4 S cm-1 For comparison : s (copper) : s (teflon) 203 ~ 106 S cm-1 ~ 10-15 S cm-1 Doping leads to enhanced conductivity Semiconductor s ~ 10-5 S cm-1 n - e- + e- Oxidation - + n Metal s ~ 104 S cm-1 204 Reduction n Electrical conductivities Copper Platinum Bismuth Graphite Doped polyacetylene is, e.g., comparable to good conductors such as copper and silver, whereas in its original form it is a semiconductor. 10+6 10+4 10+2 100 Germanium Silicon Polymers 10-2 10-4 10-6 Polyethylene 10-8 10-10 10-12 Diamond 10-14 10-16 Quartz -1 10-18 S cm The game offers a simple model of a doped polymer. The pieces cannot move unless there is at least one empty "hole". In the polymer each piece is an electron that jumps to a hole vacated by another one. This creates a movement along the molecule - an electric current 205 Examples of conducting polymers H H Polyaniline (PANI) Polypyrrole (PPy) Polyethylene dioxythiophene (PEDOT) O N N Polythiophene (PT) n N O S Polyparaphenylene vinylene (PPV) N N n S Polyparaphenylene (PPP) n n Alkoxy-substituted polyparaphenylene vinylene (MEH-PPV) O n n O 206 n Polyacetylene - electronic structure -electronic energy levels and electron occupation (a) (a) ethylene (b) allyl radical (c) butadiene 207 (b) (c) (d) (d) regular trans-PA (e) dimerised trans-PA (e) 208 p Orbitals + _ * p atom diatomic molecule …… * band band extended molecule bonds Bonding combination of p orbitals () electron density builds up between the nuclei Antibonding combination of p orbitals (*) The wavefunctions cancel between the nuclei. Larger molecules To generate the molecular orbitals of larger molecules, we take linear combinations of the atomic orbitals from each atom. If there are n atoms, there will be n different combinations. With a little bit of math, the energy levels and wave functions to the right can be found. butadiene fully antibonding 3 nodes partially antibonding 2 nodes partially bonding 1 node fully bonding 0 nodes Top down view of the p orbitals Atkins, Physical Chemistry Benzene The electrons The s electrons Top down view of the p orbitals Atkins, Physical Chemistry Conjugated polymers PA: polyacetylene (1st conducting polymer) PPV: poly(phenylene-vinylene) (used in 1st polymer LED) n S PT: polythiophene (widely used in transistors) S n PPP: poly(para-phenylene) (large bandgap) n Band diagram for poly(para-phenylene) Benzene levels n PPP Eg -/a 0 /a k E.K. Miller et al., Phys. Rev. B, 60 (1999) p. 8028. Tuning the bandgap of conjugated polymers ~ 3 eV ~ 2 eV Tuning the bandgap of polythiophene derivatives which one has the largest bandgap? Mats Andersson et al. J. Mater. Chem., 9 (1999) p. 1933. How to make conjugated polymers: 1. precursor methods The Durham precursor route to polyacetylene. The Wessling-Zimmermann route to PPV. water-soluble polyelectrolyte Feast et al. Polymer 37 (1996) p. 5017. Synthesis of PPV Synthesis of Soluble PPV CH3O i. NaOH / MeOH OH CH3O O ii. Br Dioxane 30% Formaline dry HCL ClCH2 O THF CH3O O CH2Cl CH3O Potassium tert. butoxide n / HCl Different forms of PANI Oxidative polymerization of thiophene Electrochemical mechanism How to make conjugated polymers: 2. polycondensation X X Y Commonly Used Coupling Reactions • Stille Coupling • Suzuki Coupling • Heck Reaction • Ullmann Reaction • Sonogashira Coupling • Kumada and Negishi Coupling Y [ ] n Stille Coupling Pd(II)L2Cl2 + 2R’SnBu3 R-R’ 3. Reductive Elimination R’-R’ + 2Bu3SnCl Pd(0)L2 1. Oxidative addition RX RPdL2R’ 2. Transmetalation Bu3SnX RPdL2X R’SnBu3 Suzuki Coupling Ullmann Reaction Heck Reaction Sonogashira Coupling http://www.organic-chemistry.org Pd(0) n (HO)2B B(OH)2 + n Br Br n 226 Negishi Coupling Kumada Coupling Different approaches to one polymer n-type doping Energy e- Lithium Polymer Reducing agents donate electrons to the conduction band. Solids like calcium, lithium and sodium tend to dope the polymer only near the surface since they cannot diffuse into the film. Electrolytes (see below) can be used to dope an entire film. (-polymer)n + (Na+(Naphthalide)-]y -> [(Na+)y(-polymer)-y]n + y(Naphth)0 Counter-ion Reduced polymer Oxidized molecule p-type doping Polyethylene dioxythiophene polystyrene sulphonate (PEDOT/PSS) can be bought from Bayer as an aqueous solution under the trade name Baytron. PEDOT PSS The sulphonic acid group on the PSS dopes the PEDOT to make it conductive. The Nobel Prize in Chemistry 2000 Small conjugated molecules anthracene tetracene It is not impossible to solution deposit small molecules, but it is usually hard to make high quality films because the solution viscosity is too low. Small molecules are usually deposited from the vapor phase. Mobilities of >1 cm2/V-s can be achieved by thermally evaporating thin films. The mobility is limited by grain boundaries. Mobilities > 3 cm2/Vs can be achieved by growing single crystals. pentacene Thermal evaporation of small molecules Substrate The pressure is low enough for the mean free path of a molecule to be larger than the size of the chamber. Shutter Impurities with a higher vapor pressure than the desired molecule are deposited on the shutter. q Host Heater Dopant Pump Peter Peumans Depositing polymers: Spin coating Procedure 1. Dissolve the material. 2. Cast the solution onto the substrate. 3. Spin the substrate at 1000 to 6000 revolutions per minute. Most (~ 99 %) of the solution is flung off of the substrate, but a high-quality thin film is left behind. The thickness of the films goes up with increasing solution concentration and down with increasing spin speed. Polymers with larger molecular weights tend to result in more viscous solutions, which yield thicker films. Features of spin coating Advantages • Spin coating can be done at atmospheric pressure and is very cheap. • Film thickness of up to several hundred nanometers can be obtained. • The thickness can be controlled (but not as well as with evaporation). • The thickness is fairly uniform across the substrate (except at the edge). Disadvantages • The whole substrate is coated. Patterning must be done separately. • In most cases, part of the material must be nonconjugated so that the molecules are soluble. • In can be difficult to make multilayer structures because the deposition of one layer can dissolve the layer underneath. Screen printing • This technique is used to put patterns on T-shirts. • The squeegee is used to press the dye through the screen. • Recently screen printing of polymers has been used to make LEDs and photovoltaic cells. This LED doesn’t have perfectly uniform emission, but it isn’t bad for the first demonstration of this deposition method. Ghassan Jabbour et al. Adv. Mater. 12 (2000) p. 1249. Advantages and disadvantages of screen printing Advantages • Large areas can be covered at low cost. • Atmospheric pressure. • Patterning is possible. Disadvantages • Controlling film thickness might be difficult. • The material must be soluble and have a viscosity within a certain range. Drop casting Drying solution that leaves a film behind. Petri Dish Substrate Hotplate Covering the sample with a Petri dish slows down the evaporation rate, which results in more uniform films. Keeping the substrate level results in much more uniform films. Since the solvent evaporates slowly, the material can crystallize or aggregate into fairly well ordered structures. (This can be good or bad.) Summary of drop casting Advantages • Film thickness can be far greater than 1 m. • This method is very inexpensive. • In many cases the film can be removed from the substrate by peeling or by dissolving the substrate (which would be NaCl or KBr). Disadvantages • The film thickness is difficult to control and is not very uniform. • Very thin films are difficult to make. • The material must be soluble. Ink Jet Printing (IJP) Polymers can be deposited from a printer onto a substrate. Advantages • Patterning with resolution approaching 5-10 m is possible. • No material is wasted. (~ 99 % is wasted with spin casting) • Cost can be extremely low Disadvantages • Controlling film thickness is difficult. • Fabrication of multilayer structure is difficult (compared to evaporation) Conducting Mechanism 241 How does a conducting polymer work ? Oxidative doping of polyacetylene by iodine [CH]nx+ + xI3- [CH]n + (3x/2) I2 + . I 3- + Polaron and its delocalisation . I3 - + . I 3Oxidation with iodine causes the electrons to be jerked out of the polymer, leaving "holes" in the form of positive charges that can move along the chain. 242 Excitations Bipolaron + . oxidation + + Neutral Soliton isomerisation . Positive Soliton oxidation 243 + 244 Applications of conducting polymers Polyaniline (PANI) Transparent conducting electrodes Electromagnetic shield Corrosion inhibitor ‘Smart windows’ (electrochromism) Polypyrrole (Ppy) Radar-invisible screen coating (microwave absorption) Sensor (active layer) Polythiophene (PT) Field-effect transistor Anti-static coating Hole injecting electrode in OLED Polyphenylenevinylene (PPV) Active layer in OLED 245 Organic light emitting diodes (OLED’s) An OLED is any light emitting diode (LED) which emissive electroluminescent layer is composed of a film of organic compounds. • A light emitting polymer is an electro-luminescent plastic. The molecules of this plastic emit light when an electric field is applied. • Polymer based light-emitting diodes (LED) were discovered in 1990. • Cambridge Display Technology has the core patents for the Technology of LEP. Flexible plastic serves as home to new, highly efficient organic light-emitting diodes. OLEDs provide high-contrast and low-energy displays that are rapidly becoming the dominant technology for advanced electronic screens. 246 OLED Structure Electric field Metal electrode Organic thin film + Transparent electrode (ITO) An OLED consists of the following parts: Light 1. Substrate (clear plastic, glass, foil) - The substrate supports the OLED. 2. Anode (transparent) - The anode removes electrons (adds electron "holes") when a current flows through the device. 3. Conducting layer - This layer is made of organic plastic molecules that transport "holes" from the anode. One conducting polymer used in OLEDs is polyaniline. 4. Emissive layer - This layer is made of organic plastic molecules (different ones from the conducting layer) that transport electrons from the cathode; this is where light is made. One polymer used in the emissive layer is polyfluorene. 5. Cathode (may or may not be transparent depending on the type of OLED) - The cathode injects electrons when a current flows through the device. 247 OLED Operation • A typical OLED is composed of a layer of organic materials situated between two electrodes, the anode and cathode, all deposited on a substrate. The organic molecules are electrically conductive as a result of delocalization of pi electrons caused by conjugation over all or part of the molecule •During operation, a voltage is applied across the OLED such that the anode is positive with respect to the cathode. • A current of electrons flows through the device from cathode to anode, as electrons are injected into the LUMO of the organic layer at the cathode and withdrawn from the HOMO at the anode. • This latter process may also be described as the injection of electron holes into the HOMO. • Electrostatic forces bring the electrons and the holes towards each other and they recombine forming an exciton, a bound state of the electron and hole. • This happens closer to the emissive layer, because in organic semiconductors holes are generally more mobile than electrons. •The decay of this excited state results in a relaxation of the energy levels of the electron, accompanied by emission of radiation whose frequency is in the visible region. •The frequency of this radiation depends on the band gap of the material, in this case the difference in energy between the HOMO and LUMO. 248 249 Principle of EL eLUMO LUMO h+ HOMO Cathode HOMO Anode Light Polymers for Organic Light Emitting Diodes (OLED) O n PPV n O MEH-PPV Commercial materials like Mn2+ in ZnS require 100V DC PPV : requires 5 - 10V DC runs even with AC brightness ~40,000 cd/m2 ie. ~100 times brighter than a TV screen 252 253 254 255 Renewable Technologies: Need • It is expected that the global energy demand will double within the next 50 years. • Fossil fuels are running out and are held responsible for the increased concentration of carbon dioxide in the earth’s atmosphere. • Hence, developing environmentally friendly renewable energy is one of the challenges to society in the 21st century. • One of the renewable technologies is the hydrogen fuel cell that generates electricity from the chemical reaction of hydrogen and oxygen as a byproduct water. • Similarely photovoltaics (PV), is the other renewable energy technologies is the technology that directly converts daylight into electricity. • Thus, hydrogen fuel cells and photovoltaic (PV) energy technologies can contribute to environmentally friendly renewable energy production and mitigate the issues associated to carbon dioxide emission……Global warming!!! 256 257 Molecular structures of the conjugated polymers transpolyacetylene (PA), poly(pphenylenevinylene) (PPV), and a substituted PPV (MDMO-PPV) 258 Basic processes in an organic solar cell Various architectures for organic solar cells have been investigated in recent years. In general, for a successful organic photovoltaic cell four important processes have to be optimized to obtain a high conversion efficiency of solar energy into electrical energy. -Absorption of light -Charge transfer and separation of the opposite charges -Charge transport - Charge collection For an efficient collection of photons, the absorption spectrum of the photoactive organic layer should match the solar emission spectrum and the layer should be sufficiently thick to absorb all incident light. A better overlap with the solar emission spectrum is obtained by lowering the band gap of the organic material. 259 260 The need for two semiconductors: Photovoltaic cell configurations based on organic materials differ from those based on inorganic semiconductors, because the physical properties of inorganic and organic semiconductors are significantly different. • Lower dielectric constant • Exciton binding energy is larger than inorganic semiconductors energy (for GaAs the exciton binding energy is 4 meV, for polydiacetylene 0.5 eV) • Dissociation into free charge carriers does not occur at room temperature in case of organic conducting polymers. Hence, low mobility of the charge carriers and the internal field of the p-n junction. Both p-type (donar) and n-type (acceptor) material is required for organic PV technology 261 262 263 264 Schematic drawing of the working principle of organic photovoltaic cell 265 266 Bulk-heterojunction solar cells Power conversion efficiencies exceeding 2.5% (MDMO-PPV as a donor and PCBM as an acceptor) 267 The bulk-heterojunction concept. 268 269 270 ITO substrate Pattern generated in Aqua regia PEDOT PSS layer Spin coated Polymer +PCBM deposition by spin coating Deposition of metal cathodes by thermal evaporation BHJ PEDOT PSS layer Spin coated V Bilayer V polymer layer Spin coated 271 PCBM layer Spin coated Deposition of metal cathodes by thermal evaporation 272 273 165,000 TW of sunlight hit the earth 274 275 276 277 278 Optimizations at Spatial Domain 279 280 281 282 BTFM-PPV BTFM/MEH-PPV DBTFM-PPV F F F CF3 CF3 CF3 OCH3 O 283 n O x n y O F3C F Introduction The global energy demand expected to be double within the next 30-40 years 90% of present global energy is supplied by fossil fuels •Non-sustainable •Negative environmental impacts Need sustainable, environmentally-friendly energy sources Fuel cells are a promising technology Introduction PEMFCs are considered as reliable alternate energy converting device because of high power density high efficiency low operating temperatures solid non-corrosive electrolyte long life ease of design and adaptable size environmentally friendly Polymer electrolyte membranes (PEMs) are key materials for proton exchange membrane fuel cells (PEMFCs) Applications: Automobiles portable devices Stationary systems Working Principle of PEMFC 1. When hydrogen is lead to the first catalyst layer, at anode, the hydrogen molecules are split into their basic elements, i.e. protons and electrons. 2. The protons migrate through the electrolyte membrane to the second catalyst layer, the cathode. Here they react with oxygen to form water. 3. At the same time the electrons are forced to travel through external circuit to the cathode side, because they can not pass the membrane 4. This movement of electrons thus creates an electrical current Electrode reaction: Anode: H2 2 H+ + 2 e- Cathode: ½ O2 + 2 H+ + 2 eOverall: 2H2 + ½ O2 catalyst H2O H2O Proton transport mechanism “Grotthus mechanism” •Protons hop from one hydrolyzed ionic site (SO3¯H3O+ ) to another across the membrane • It involves conversion of H-bonds to covalent bonds between water molecules and vice versa; only the charge of the proton is transported and not its mass. “Vehicular mechanism” • Hydrated proton (H3O+) diffuses through the aqueous medium in response to the electrochemical difference • The water connected protons (H+ (H2O)x) in the result of the electroosmotic drag carry the one or, more molecules of water through the membrane and itself are transferred with them. Scope of the Work Required and desirable characteristics High ionic conductivity (and zero electronic conductivity) Good mechanical strength Long-term chemical stability i.e. good oxidative and hydrolytic stability Good thermal stability Low oxidant and fuel cross-over Low cost and ready availability DuPont's Nafion®: A state of art PEM material for fuel cell Nafion®117 : m ≥1, n=2, y=1, x=7-20 Advantage: Disadvantage: Excellent Proton Conductivity up to 10-2-10-1 S/cm Good chemical & oxidative stability Long term stability Expensive (up to US$ 700 per square meter). Nafion® High methanol permeability Loss of membrane performance at elevated temperature (>80 oC) The current state-of-the-art performance is not high enough for the practical applications Why does Nafion® possess high proton conductivity? nano scale phase separation between hydrophilic and hydrophobic domains connectivity between the hydrophilic domains Hydrocarbon based alternative PEM materials Sulfonated PEEK Sulfonated Polyimide Sulfoarylated PBI Sulfonated Poly(arylene ether sulfone) Sulfonated Poly[(3methylphenoxy)(phenoxy)phosphazene] HO OH A A n n n n B 0.1n 0.2n 0.3n 0.4n C 0.9n 0.8n 0.7n 0.6n O Cl S Cl O B HO3S SO3H O Cl S C Cl O 291 General understanding about hydrocarbon based PEM materials Increase in IEC results in increase in proton conductivity. A trade-off relation exists between IEC and stability related properties (like dimensional stability, hydrolytic- oxidative stability) & also with mechanical strength. Control of polymer morphology and ionic nanostructure is effective to obtain PEMs which have moderate IEC values and high proton conductivity. Methodologies to obtain desired properties Introduction of polar and flexible ether linkages to have better solubility & processability. Inclusion of hydrophobic pendant -CF3 groups to have better phase separated morphology and stability. Copolymerization properties. to control IEC and other Synthesis of semifluorinated 5-Membered SPIs HO3S H2 N Scheme : NH2 SO3H DSDSA Synthesis of diamine monomer N(CH2CH3)3 N2 flow H2N X NH2 + m-cresol/ 80oC + H2N O NH2 SO3H N+ QA O O H N- + O3 S O O O O O BPADA N2 flow m-cresol/ 800C /4 hr Sulfonated polyamic acid as triethylammonium sulfate form N2 flow Quadri diamine(QA) 1800 C/ 16 hr 2000C/ 4 hr -(m+n) H2O O * N Ref: S. Banerjee et al. / Polymer 44 (2003) 613–622 O N N X O O N N X H N+ O3 S * SO3N+ H m O O 1-m O O O N O O O O O Acid treatment Yield= 99 % * N O 1-m O O O O O O O O N O HO3S SO3H * m Sulfonated Polyimide CF3 X= O O F3C E.A. Mistri, A.K Mohanty, S. Banerjee / Journal of Membrane Science 411– 412 (2012) 117– 129 m = 0.5, (DSDSA/QA) = 0.6, (DSDSA/QA) = 0.7, (DSDSA/QA) = 0.8, (DSDSA/QA) = 0.9, (DSDSA/QA) = 1.0, (DSDSA/QA) = 50/50 = 60/40 = 70/30 = 80/20 = 90/10 = 100/0 Good thermo-mechanical properties 70 100 DRY Membranes 60 Tensile stress (MPa) Weight (%) 80 60 DQB50 DQB60 DQB70 DQB80 DQB90 DQB100 40 20 50 40 30 DQB50 DQB60 DQB70 DQB80 DQB90 DQB100 20 10 0 0 0 100 200 300 400 500 600 700 800 900 0 5 Temperature (oC) 10 15 20 25 Tensile strain (%) TGA plots of the SPIs; heating rate: 10 oC min−1 (Stress-strain plot of DQB membranes; strain rate 5%/min, at 25 oC and 65% RH) Initial weight loss ~ 100 ºC evaporation of the absorbed water. nd 2 weight loss ~ 285 ºC decomposition of sulfonic acid groups. 3rd weight loss around 580 ºC -600 ºC pyrolysis of the SPI backbones. Good mechanical properties (tensile strength ranging from 42 – 66 MPa and Young’s moduli of 1.45–1.67GPa). Microstructure, IEC & proton conductivity DQB 50 DQB 80 DQB 70 DQB 60 TEM micrographs of SPI membranes (cross section, Ag+ form) Polymer membranes DSDSA mole % IEC (meq/g) Theo. Exp. WU (wt %) Proton conductivity (S.cm-1) DQB50 50 1.00 0.96 14.0 3.96×10-3 DQB60 60 1.24 1.19 20.55 1.07×10-2 DQB70 70 1.49 1.42 25.29 1.47×10-2 DQB80 80 1.75 1.65 30.0 2.09×10-2 DQB90 90 2.04 1.93 35.17 3.45×10-2 DQB100 100 2.34 2.20 41.27 – Proton conductivity, s (mS/cm) PEM properties : 35 30 High Proton conductivity up to 34.5 mS/cm 25 20 15 10 At 30 °C and 100% RH 5 0 0.8 1.0 1.2 1.4 1.6 1.8 2.0 IEC (meq/g) Proton conductivity of SPIs as a function of IEC Limitation: Suffers with poor hydrolytic stability O The major concern of the synthesized sulfonated polyimide membranes is that they suffer from hydrolytic degradation in acidic medium during acid treatment and subsequently all the membrane becomes brittle after a certain time. N O HO3S +H HO3S + H2O N SO3H OH2 HO OH HO3S N SO3H O SO3H O -H / +H O C OH C NH OH HO3S NH HO3S -H / +H SO3H OH HO OH C OH HO3S C NH O SO3H O SO3H + H2 O O C OH C NH H2O OH -H / +H HO H2N H2 N C SO3H HO3S O C OH HO3S OH HO3S SO3H SO3H -H + O C OH Mechanism of hydrolytic degradation Imide Acid- amide Carboxylic diacid DQB90 (1N HCl treated) DQB80 (1N HCl treated) Acid-hydrolysis of 5-membered imide ring leads to chain scission and subsequently, DQB70 (1N HCl treated) DQB60 (1N HCl treated) Membrane becomes brittle Mw of the polymer decreases DQB50 (1N HCl treated) C OH O Studies on semifluorinated 6-Membered SPIs O O N N O O O CF3 O O O N N O O 1-m F3C DQN XX series HO3S SO3H m=0.4, DQN 40 =0.5, DQN 50 =0.6, DQN 60 =0.7, DQN 70 =0.8, DQN 80 =0.9, DQN 90 • Possess good solubility in common organic solvents • High molecular weight • Excellent thermal and mechanical properties • Good dimensional stability with decent hydrolytic-oxidative stability • Nano-scale phase separated structure • High proton conductivity. m Physical Properties Thermal Mechanical 100 100 Weight (%) 80 1 2 3 4 5 6 60 40 DQN40 DQN50 DQN60 DQN70 DQN80 DQN90 Tensile Stress (MPa) Dry Membranes 1 6 54 2 3 20 heating rate: 10 °C min-1 ; in air) 0 100 200 300 400 1 3 80 4 5 60 40 Excellent mechanical properties (tensile strength ranging from 65.4–105.6 MPa and Young’s moduli of 1.56–2.0 Gpa) 20 0 2 1 2 3 4 5 DQN40 DQN50 DQN60 DQN70 DQN80 0 500 600 0 700 10 20 30 40 50 Tensile Strain (%) Temperature (oC) Stress-strain plot of DQN membranes TGA thermograms of the DQN polymers; Chemical stability Polymer membrane DQN40 Hydrolytic stability a (h) >250 Oxidative stability b (h) τ1 τ2 3.8 >24 DQN50 >250 3.5 >24 DQN60 >250 3 20 DQN70 >250 1.2 9 DQN80 ~120 0.8 4.5 a Time that the membrane is completely dissolved in deionized water at 80 °C. b Tests performed in Fenton’s reagent at 80 °C. τ and τ are the 1 2 elapsed times in which the membranes start to break and dissolve completely.. Microstructure IEC and proton conductivity • Excellent nano phase separated structure • High proton conductivity in the range of Nafion at 100 % relative humidity 16000 DQN40 DQN50 DQN60 DQN70 DQN80 DQN90 14000 DQN 80 12000 Z" (Ohm) DQN 70 DQN 60 DQN 50 TEM micrographs of SPI membranes (cross section, Ag+ form) • 10000 8000 6000 4000 PEM properties: 2000 0 Arrhenius equations, σ = Ae-Ea/RT 5000 10000 15000 20000 25000 30000 35000 Z' (Ohm) 90 5.0 80 DQN40 DQN50 DQN60 DQN70 DQN80 DQN90 4.5 70 O 4.0 -1 ) at 30 C 60 50 ln s (mS. cm Proton Conductivity (mS/cm) 0 40 30 20 10 Variation in the impedance behaviour Polymer membrane IEC (meq/g) 3.5 Proton conductivity (mS cm-1) 30 °C 80°C Ea (kJ/mol) theo.a exp.b DQN40 1.03 0.98 5 9.4 11 DQN50 1.34 1.36 6.5 13.2 12.2 DQN60 1.67 1.67 7.6 17 14.6 DQN70 2.03 n.d. 22.8 39.6 8.9 DQN80 2.42 n.d. 54.5 80.6 7.2 DQN90 2.85 n.d. 81.9 108.2 4.6 3.0 2.5 2.0 0 1.0 IEC 1.5 theo 2.0 (mm ol/g) 2.5 3.0 10 15 20 W 25 r at e 30 Up 35 t 40 45 (w ake Correlation plot of IECtheo, WU and proton conductivity ) t. % 1.5 2.8 2.9 3.0 3.1 3.2 3.3 3.4 -1 1000/T (K ) Temperature dependence of proton conductivity Studies on semifluorinated sulfonated poly(arylene ether sulfone)s Reaction scheme: n Cl NaO3S O S O SDCDPS SO3Na Cl CF3 CH3 + OH + (1-n) F HO CH3 BPA F F3C QBF K2CO3 NMP 180 oC O S O SO3Na CF3 CH3 O O CH3 NaO3S n F3C HO3S SO3H BPAQS-xx O CH3 hydrophilic CF3 CH3 O O CH3 H+ O S O CH3 O n XX 0.2 0.3 0.4 0.5 0.6 20 30 40 50 60 CH3 O n F3C (1-n) O CH3 (1-n) hydrophobic BPAQSH-xx Approach to the structural designing: •QBF is incorporated to control the DS of the random copolymer BPAQSH-xx •-CF3 group in QBF increases the hydrophobicity of non-sulfonated backbone segment over sulfonated segment which may help for good phase separated morphology •SDCDPS with features of localized sulfonic acid density and rigid QBF moiety are incorporated into the backbone structure of polymer to increase the statistical length of non-sulfonated segment which may help to obtain good mechanical property, high hot water stability and high proton conductivity. •rigid structure with pendant -CF3 group of QBF moiety will help to attain good solubility, thermal, oxidative stability Thermal properties, mechanical properties and oxidative stability Polymer Tga (/oC) Td5% b Td1 b (/oC) Td2 b (/oC) TS (/Mpa) Y (/GPa) EB (/%) Oxidative stability, tc (/h) (/oC) BPAQ 216 476 431 - 40 1.04 59 - BPAQSH-20 245 365 393 546 57 1.55 18 29.6 BPAQSH-30 257 355 389 533 49 1.25 21 25.8 BPAQSH-40 ND 347 368 528 37 1.05 19 4.5 BPAQSH-50 ND 310 366 526 35 1.02 18 4.3 BPAQSH-60 ND 283 261 512 27 0.91 4 3.8 a glass transition temperature determined by DSC, heating rate 10 oC/min under nitrogen. b td refers to decomposition temperature measured by TGA, heating rate 10 oC/min under air. c ‘t’ refers to the time expended for the membrane beginning to break in Fenton’s reagent (2ppm FeSO4 in 3% H2O2) at 80 oC. Microstructure and PEM properties BPAQS-30 BPAQS-40 BPAQS-50 TEM micrograph of lead-stained BPAQS-XX copolymers BPAQS-60 PEM properties: Polymer ηinha (dL/g) IECW (meq/g) WUW (%) σ (mS/cm) Swelling (%) Titr. 30 oC 80 oC 30 oC 80 oC 30 oC 80 oC Eab (KJ/mol) xx=20 1.28 0.58 4 7 2.2 2.6 9 25 17.26 xx=30 1.25 0.84 7 12 2.8 3.3 13 31 16.44 xx=40 1.23 1.18 12 18 4.0 5.3 16 40 15.72 xx=50 1.21 1.46 22 36 4.6 6.3 31 71 13.96 xx=60 1.19 1.70 40 52 6.2 10.2 40 80 11.34 a inherent viscosity of BPAQS-XX copolymers in NMP at 30 °C; energy determined in the temperature range: 30-90 oC and heating rate 1-2 oC/min. b activation PEM properties 100 for BPAQSH-XX copolymers o 80 C 50 40 o 30 C 30 20 10 0.6 0.8 1.0 1.2 1.4 1.6 1.8 for BPAQSH-XX copolymers 80 60 40 Inflection point 20 0 0.4 0 2.0 o at 30 C o at 80 C 0.6 5.0 σ = Ae-Ea/RT 1.0 1.2 1.4 1.6 1.8 Proton conductivity as a function of IEC Water uptake as a function of IEC Arrhenius equation: 0.8 IECw (meq/g) IECw (meq/g) (I)BPAQSH-20 (II)BPAQSH-30 (III)BPAQSH-40 (IV)BPAQSH-50 (V)BPAQSH-60 4.5 4.0 V Ln(smS/cm) Water uptake (wt. %) 60 Proton conductivity, s (mS/cm) 70 3.5 IV 3.0 Arrhenius temperature dependence of proton conductivity (σ) of BPAQSH-XX membranes III II 2.5 I 2.0 2.7 2.8 2.9 3.0 3.1 1000K-1/T 3.2 3.3 3.4 Summary Both the synthesized aromatic SPIs and poly(arylene ether sulfone)s draw special attention as high performance polymeric materials with ease of processability and possessing an excellent balance between thermal , mechanical properties and proton conductivity. The synthesized 5-membered SPI (DQB XX) membranes are hydrolytically unstable and all the membranes undergoes hydrolysis upon long treatment with acid solution or long term keeping in water at room temperature but the 6-membered SPI (DQN XX) possess excellent hydrolytic-oxidative stability. Introduction of ether linkages (―O―) and bulky (―CF3) pendent group into polymer chain resulted in increase in many physical properties like solubility, processability, dimensional stability and chemical stability. Hydrophobic (―CF3) group also helps to form well phase separated structure responsible for better proton conductivity. High proton conductivity have been achieved for the membranes having moderately high IEC (88mS/cm at 90 oC for BPAQSH-60 and 108.2 mS/cm at 80 oC for DQN-90 sample). Overall combination of good PEM properties renders BPAQSH-50, BPAQSH-60 and DQN-70, DQN-80 as potential candidate for use as PEM materials. 306

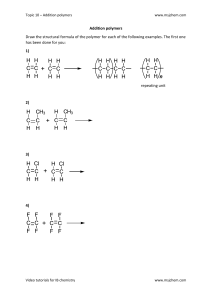
![\t<L Your Name: _[printed]](http://s2.studylib.net/store/data/013223479_1-5f2dc062f9b1decaffac7397375b3984-300x300.png)