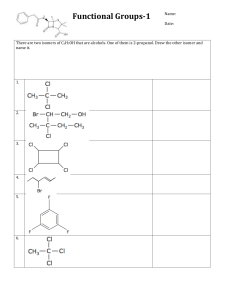
Revision exercises Exercise 1 The complete combustion of 7.4g of an alcohol (A) gives 17.6g of carbon dioxide. 1. Write the complete combustion equation for an (A). 2. Determine the molecular formula of (A). 3. Give the condensed structural formula, the name and the class of all the isomeric alcohols corresponding to this formula. 4. The mild oxidation of (A) gives a compound (B) which reacts with D.N.P.H and does not react with the sciff reagent. a- Identify the alcohol (A), justifying the answer. b- Give the condensed structural formula of (B) and its name. Exercise 2 Hydration of an alkene (A) gives a compound (B) of molar mass M = 46g.mol-1. 1. a- Write in condensed structural formula, the equation of this reaction. a- Determine the gross formula of (B) and that of (A). 2. The following experiments are carried out: • 1st Experiment: To a solution of (B) is added a solution of potassium dichromate and a few drops of sulfuric acid. • 2nd Experiment: The solution is heated (B). a- What is the reaction for each reaction? b- Write the equations of the reactions carried out. Exercise 3 1. We consider an alcohol (A) of molar mass M = 60 g.mol-1. Determine its raw formula. Give its isomers and the name of each. 2. The mild oxidation of the primary alcohol isomer of (A) is carried out with oxygen from the air and the mild oxidation of the secondary alcohol using a solution of potassium dichromate (2K +, Cr2O72-) in the medium acid. a- How can we experimentally identify the products obtained in each experiment. b- Write the equations of the reactions occurring in each experiment. Give the names of the products obtained. Exercise 6 The complete combustion of 0.37 g of an alcohol (C4H10O) requires a volume V = 0.72 L of oxygen under the temperature and pressure conditions where the molar volume of the gases is equal to 24 L.mol-1. 1. a- Write the complete combustion equation for an alcohol (A). a- Give the condensed structural formula, the name and the class of all the isomeric alcohols corresponding to this formula. 2. The controlled oxidation of (A) is carried out with the oxygen in the air, a compound (B) is obtained which reacts with D.N.P.H and which turns the sciff reagent. a- Identify the alcohol (A) knowing that its position isomer does not react during mild oxidation. b- Give the condensed structural formula of (B) and its name. c- The oxidation of (B) gives a compound (C), give the name and the condensed structural formula of (C). 3. The dehydrogention of the alcohol is carried out at a temperature of 300 ° C. a compound (D) is obtained which gives orange yellow precepitate with DNPH and blue color with fehling. .a- Write the equation of the reaction. c- Give the family, the name and the condensed structural formula of (D).