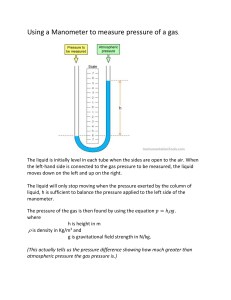
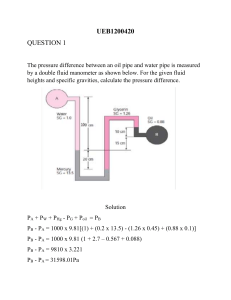
There’s no need to memorize cubed or squared conversion factors gc = Notes on CheCal 1 with input Collab on 08/12/21 Difference of Units and Dimensions: o Units: standard way of describing physical quantity o Dimensions: physical quantity Conversion Factor: gives the relationship of the two units involved; in fraction form SI Units: o Greek prefixes o In powers of 10 o Base unit + Greek prefix (also called multiplier) Format for solution: o Given, required, solution: conversion factors then solution For answers: o Usually 5% difference or deviation from calculated answer for engineering students but only 0.1% for sir “No particular rule for rounding off”, as long as it is reasonable and not too long **use exponential form if possible to be safe Exponential and logarithmic shouldn’t have units gc is a conversion factor to relate mass and force **it’s referred to as the “C” in Himmelblau’s where it is defined as a constant with a value of one to balance the units and is dependent on the units of the dimensions used in the equation Dyne: is force used for small objects In writing exponential form: x 10n as en 1 𝑔∙𝑐𝑚/𝑠2 𝑑𝑦𝑛𝑒 32.174 𝑙𝑏𝑚 ∙𝑓𝑡/𝑠2 = 𝑙𝑏𝑓 1 𝑘𝑔∙𝑚/𝑠2 𝑁 = Not on collab notes: 1 Btu = 3.93 x 10-4 hp-hr Collab on 08/17/20 Density as conversion factor o 1 g/cm3 = 62.4 lb/ft3 o 1 cP = 0.01 g/cm•s For dimensional analysis/consistency; changing the units of the components: o Do it reverse, use the goal units then convert back to the original unit –new units back to original units o Work only on one side of the equation o The resulting unit of the equation is added at the end and will be converted to the desired/goal unit Try checking the method in SIM, the one that tackles temperature etc. Collab on 08/19/21 Mole unit o Amount of particles (molecules, atoms, electrons) o 0.012 kg of C-12 o o o o o o 𝑛= 𝑚 𝐹𝑊 = 12 𝑔 12 𝑔/𝑚𝑜𝑙 1000𝑔 1 𝑘𝑔 = 12 𝑔 𝑚 𝑖𝑛 𝑙𝑏 𝑀𝑊 𝑖𝑛 𝑙𝑏/𝑙𝑏𝑚𝑜𝑙 𝑚 𝑖𝑛 𝑔 1 𝑔𝑚𝑜𝑙 = 𝑀𝑊 𝑖𝑛 𝑔/𝑔𝑚𝑜𝑙 𝑚 𝑖𝑛 𝑘𝑔 1 𝑘𝑔𝑚𝑜𝑙 = 𝑀𝑊 𝑖𝑛 𝑘𝑔/𝑘𝑚𝑜𝑙 1 𝑙𝑏𝑚𝑜𝑙 = Also kgmol Density o Mass of substance per unit volume o Kg/m3, lb/ft3, g/mL, g/cm3 Specific volume is reciprocal of density Density of water, at 80-100 temperature, density changes (it goes down) Relative density = specific gravity SG o A universal reference scale o Has two major drawbacks: Laboratory precision Limited range of scale o Often expressed to an accuracy of 2 or 3 decimal places Reference density for SG: o For liquids and solids, water o For gases, air Comparing the density-temperature graph of NH3 and water, the former has apparent changes in density depending on the temperature Solutions assume no changes in density as temperature changes 𝑆𝐺 = 𝜌𝑠𝑢𝑏𝑠𝑡𝑎𝑛𝑐𝑒 𝜌𝑟𝑒𝑓𝑒𝑟𝑒𝑛𝑐𝑒 Usual density used as reference for solids and liquids is density of water at 4˚C >> 1.00 g/cm3 𝑆𝐺 = 0.72 = 1𝑚𝑜𝑙𝐶 Etymology > mole > Latin > heap Amount of substance 1 mole = 6.022x1023 atoms/molecules/ions Avogadro’s number of elementary units/entities 𝑚𝑐 = 0.012 𝑘𝑔 × o o ˚API o 20° 4° Numerator: temperature of substance when measured Denominator: temperature of reference when measured American Petroleum Institute 141.5 o °𝐴𝑃𝐼 = o Hydrometric scale based on petroleum products Measure of the density of petroleum liquid in relation to density of water Gravity or density of crude oil and liquid petroleum products Devised by American Petroleum Institute and NIST – National Institute of Standards and Technology Oil with the least specific gravity has the highest API gravity API gravity is directly proportional with temperature (SG is inversely proportional to T) Volume of petroleum liquid is directly proportional to temperature Crude oil quality standard adopted worldwide Indicate oil quality One of the important factors in deciding the price of various varieties of crude oil ˚API > 45, extra light crude oil ˚API (33,45), light crude oil o o o o o o o o o o 𝑆𝐺 60℉ 60℉ − 131.5 o o o o ˚API (22, 33), medium crude oil ˚API (10, 22), heavy crude oil ˚API ≤ 10, extra heavy crude oil Light crude oil is more expensive than heavy crude oil Higher percentage of gasoline and diesel fuel Baumé scale o ˚Be o °𝐵𝑒 = 145 − o °𝐵𝑒 = °𝑇𝑊 = o Scale for SG of solutions, first two digits to the right of the decimal point multiplied by 2 Reports the measured SG of a liquid relative to water Only used for liquids with SG greater than that of water Usually ranges between 1.00 and 1.85 Used in the British dye and bleach manufacturing industries W. Twaddell Glasgow/ Thomas Twaddell > coined after Charles Macintosh > person behind Used philosophical bubbles or SG beads Used in heavy industries to determine strength of solution of various substances such as brine, sugar solutions (syrup, juice, honey, brewers, must) and acids Scale little known outside of UK, mainly in England and Scotland Used for acids in tanning industry and alkaline lye in papermaking Uses: Tanning industry Determine strength of synthetic tanning agents Easier to determine the extra water needed to dilute mixture to desired o o 145 𝑆𝐺 Heavier than water 145 𝑆𝐺 o − 130 Lighter than water Some sources say it’s 140/SG o Antoine Baumé o Devised for making hydrometers o Used to determine the sugar content of must o Used to calculate what the wine’s alcohol content will be after fermentation o Used as a measure of density or concentration o SG of a sugar solution has a good correlation to sugar concentration In brewing >> wine making and sugar beet processing o A traditional description of a solutions concentration o A number of acids has its concentration measured in ˚Baume >> also called heaviness scale o Strength of acids is expressed by their relative weight Twaddell scale o ˚TW 𝑆𝐺−1 0.0005 o o o o o o o o strength Twaddells popular 𝐸𝑥𝑡𝑟𝑎 𝑤𝑎𝑡𝑒𝑟 𝑛𝑒𝑒𝑑𝑒𝑑 = 𝑉 in > 𝑇𝑤 𝑑𝑒𝑠𝑖𝑟𝑒𝑑 𝑠𝑡𝑟𝑒𝑛𝑔𝑡ℎ Pottery industry Calculating the volume to add given the concentration of sodium silicate (deflocculant) 1𝑔 𝑠𝑢𝑔𝑎𝑟 o 1°𝐵𝑥 = o Uses refracometer Light bends more in a substance with higher sugar content Interchanged with degrees Plato (˚P) and degrees Balling Expresses the weight percentage of sugar solutions and relate it to SG Measure of sugar content Measure of all solutes in a beverage or preparation o o o 𝑇𝑤 𝑜𝑟𝑖𝑔 𝑠𝑡𝑟𝑒𝑛𝑔𝑡ℎ−𝑇𝑤 𝑑𝑒𝑠𝑖𝑟𝑒𝑑 𝑠𝑡𝑟𝑒𝑛𝑔𝑡ℎ Brix Scale o ˚Bx o °𝐵𝑥 = 231.61(𝑆𝐺 − 0.9977) o Measurement in percentage by weight of sucrose in pure water solution Only valid for pure sucrose solutions > extracted from sugarcone or sugar-beet o Measures the sugar content in a solution Allows to estimate alcohol content of product (wine making) o 100𝑔 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 = 1% 𝑠𝑢𝑔𝑎𝑟 Other scales: Oechsle or ˚Oe (wine making) and Plato or ˚P (brewing industry) Above are SG-based scales Mass flow rate is ṁ Collab on 08/24/21 Unless stated, always use reference density (density of water) at 4˚C Make calculations as simple as possible Solve problems more easily Empirical formula o Formula that doesn’t make sense dimensionally o Used/based on experiments/ empirical data Do not overthink solutions Mole and mass fractions are mostly used in CHE calculations Concentrations such as molality, molarity, and normality are rarely used in CHE and are for smaller amount of substances Rules for percent composition: o For gases, if not stated: it’s either mole% or volume% At the same temperature and pressure, mole% = volume% o For solids and liquids, if not stated: it’s either mass% or weight% Collab on 08/26/21 Exam coverage o Conversion of units o Dimensional consistency o Mole concept o Density o Mole and mass fraction Exam details: o 1:30-3:30 o Problem solving o Write the problems in the solution paper o 2h Mass and mole fraction o Usually requires 4 decimal places “Ash” o Not necessarily ash o Non-combustibles o Residual substances that did not evaporate/ combust in the process o Usually minerals For solving average molecular weights, use the mass/mole based on the calculations as per basis Main vinculum must always be the longest In solving problems, where mole/mass of the solution is given as well as the mass % and mole % of the components: o One component as mass% o The other as mole% o Solve for the equations for the components’ mole and mass o Assign variable to x to the not given % If mole % of A is given, assign mole of B as x If mass % of B is given, assign mass of A as x o Then solve for the variable using the equations formed Collab on 09-07-21 Collab on 9/02/21 Basis o o o o o o o o Reference chosen for calculations Starting amount for calculations Explicitly stated at the beginning of the solution Assumed values Must be complete with label Prefers the most convenient basis May be a period of time, mass of material, and other convenient quantity 1 or 100 is usually the best unit basis Sun-mon, quiz Temperature o How hot/cold a body is o Defined in thermodynamics as the average kinetic energy of the atoms and molecules in the system o Usually used along with pressure to determine the properties of different substances for various systems o There are 4 scales commonly used in the field of chemical engineering -40˚ is the temperature when Celsius is equal to Fahrenheit Standard temperature is 0˚C Absolute scale o Reference is absolute zero Lowest possible temperature o Best fit for physical quantities computation o Kelvin Measures the absolute zero, 0 K Absolute scale that corresponds to Celsius William Thompson, Lord Kelvin o Rankine Absolute scale that corresponds to Fahrenheit William John Macquorn Rankine Relative scale o Celsius Measured freezing point of water as well as water’s boiling point; then divided the middle in 100 (equal parts) Anders Celsius o Fahrenheit Measured the freezing point of water, normal body temperature (96.6 ˚F and 32 ˚F) Measured freezing point of brine solution (salt solution) Daniel Gabriel Fahrenheit o Based on relative temperature of freezing and boiling point of substances o Relatively assigned Formula: o 𝑇℃ = (𝑇℉ −32) 1.8 o 𝑇℉ = 1.8𝑇℃ + 32 o 𝑇𝐾 = 𝑇℃ + 273 o 𝑇°𝑅 = 𝑇℉ + 460 For Kelvin, it should be 273.15 and Rankine should be 459.67 Temperature difference o Useful in calculations since most basis for formula is temperature difference o Derivation: ∆𝑇℃ = 𝑇℃2 − 𝑇℃1 𝑇℉ − 32 𝑇℃ = 1.8 𝑇℉2 − 32 𝑇℉1 − 32 ∆𝑇℃ = − 1.8 1.8 1.8∆𝑇℃ = 𝑇℉2 − 𝑇℉1 1.8∆𝑇℃ = ∆𝑇℉ o Used for conversion of units of constants in equations For formula equations, temperature difference is used Relation between temperature scales o ∆℃ = ∆𝐾 o ∆℉ = °𝑅 o ∆℃ = 1.8∆℉ o ∆𝐾 = 1.8∆°𝑅 Rankine still is expressed in degrees. Only Kelvin does not have degrees F˚ : temperature difference in temperature ˚F : just temperature measure 0˚C is the standard conditions of temperature Unit degree Celsius and Kelvin are larger than unit degree Fahrenheit and Rankine Collab on 09-09-21 Exam on Thursday o o o Synchronous 2:30-4:30 (1 and 45 mins) Coverage: Choosing a basis Temperature and pressure Relating scales to each other o 3-4 times o GMeet and SEB o Copy the problem o “Plan while copying the problem” o Restrict access (permits) o SEB password is given in GMeet Most equations are based on temperature difference “square of a binomial” o For squared scales in formula o Do not distribute o It’s fine if another term is added Temperature scale in first degree Pressure o Exerted on top of cylinder by atmosphere and at the bottom of the cylinder by water/substance o Force applied perpendicular to surface or the normal perpendicular force per unit area o SI unit is Pascals, Pa which is N/m2 o We will only consider pressure exerted by gases and liquids o Exerted force per area o Fluid has weight, weight will be the force exerted at the bottom of the weight o o o o o o o Discovery stemmed from welldiggers wondering why water won’t rise beyond 10 m using suction pumps They first sought Galileo but he did not want to be bothered lol Torricelli was the one who helped them From Torricelli’s experiment: Water is not pulled by vacuum but pushed up by local air pressure Therefore, maximum height of water that can’t be pumped depends on atmospheric pressure Hydrostatic pressure Pressure exerted by liquid Pressure of fluid at equilibrium Pressure at the bottom of the static (nonmoving) column of mercury exerted at the bottom/sealing plate Derivation 𝑓𝑜𝑟𝑐𝑒 𝑚𝑔 𝑃= = 𝑎𝑟𝑒𝑎 𝑎𝑟𝑒𝑎 (𝜌𝑣)𝑔 𝜌(𝑎𝑟𝑒𝑎)(ℎ)𝑔 = = 𝑎𝑟𝑒𝑎 𝑎𝑟𝑒𝑎 𝑃 = 𝜌𝑔ℎ Gravity used is usually gc, which is the conversion factor Atmospheric pressure Pressure exerted by air or atmosphere The barometric pressure 𝑃𝑡𝑜𝑡 = 𝜌𝑔ℎ + 𝑃𝑎𝑡𝑚 o o Torricelli’s Experiment Fig 1 o Patm pushes down liquid on basin and pushes up liquid to the tube o o o o o Set-up: Basin filled with mercury A tube with a close top and open bottom at the center Pressure exerted by the atmosphere will cause mercury to rise in the tube The height of the rise in mercury (fluid) is the measured atmospheric pressure The height of the fluid is 760 mm Can be expressed in either absolute or relative scale Relative pressure is not the used term, it’s called “gauge pressure” or pressure gauge instead Gauge pressure Does not include Patm Absolute pressure Patm 𝑃𝑎𝑏𝑠 = 𝑃𝑔𝑎𝑢𝑔𝑒 + 𝑃𝑎𝑡𝑚 19.3 psia (referred to zero absolute pressure) 4.6 psig (referred to barometric pressure) Standard pressure 101.3 kPa, 1.000 atm, 760 mmHg Use 1 atm as Patm if it is not given since it is the standard atmospheric pressure at sea level Head of a liquid A liquid column Head >> height of the column of liquid Collab on 09/13/21 Exam coverage o Temperature o Temperature conversion o Homogeneity of an equation o Equations involving (relating) temperature o Pressure Pressure o Perpendicular force per area For fluids/ liquid o 𝑃 = 𝜌𝑔ℎ o H : height of the liquid o Gravity is usually in g/gc Because we essentially need to convert mass to force in the equation/formula o 𝑔 𝑃 = 𝜌 (𝑔 ) ℎ 𝑐 Easier especially of English units are used SI: g/gc = 9.81 N/kg AE: g/gc = 1 lbf /1 lbm g/gc values are from values of g divided by gc Force exerted by fluid o 𝐹𝑓𝑙𝑢𝑖𝑑 = 𝑚𝑓𝑙𝑢𝑖𝑑 𝑔 = 𝜌𝑉𝑔 Absolute pressure o The sum of pressure exerted by fluid and pressure exerted by atmosphere o 𝑃𝑎𝑏𝑠 = 𝑃𝑔 + 𝑃𝑎𝑡𝑚 In exams, it will be explicitly stated if the system is open to the atmosphere or not It is easier and convenient to use g/gc in English units Manometer o Device used to measure the pressure of a sample gas o U-shaped tube filled with fluid (manometer fluid) Due to Patm, there is a difference in height of liquids Due to difference in pressure o Manometer fluid Should be completely immiscible to other fluid Creates a distinct layer that separates the liquid o If both manometer fluid at both side have equal height no reading fig. 2 o If PA > PB Results to difference fig 3 x-h x h h : difference of pressure x : height of fluid at the left from the opening to o o o o the liquid/ reference line x-h : height of fluid at the right from the opening to the reference line Used to measure pressure difference Sensitive to pressure of other fluids cheaper Can be : Open-end Measures relative pressure/ relative with Patm Relative/ gauge pressure Fig 6 Fluid is accounted with Patm Close-end Also called absolute pressure manometer The measurement is not compared with Patm Fig 7 Only force is from fluid Take note when to add Patm and when not It’s easier to create open-end than closeend manometers Bourdon Gauge o Used by water companies o Useful for high pressure readings o Used special (???) o Used on water systems Pressure Balance o Pressure on the left side of the U-tubed manometer must be equal to the pressure on the right side 𝑃𝑡𝑜𝑡 = 𝑃𝑒𝑥𝑡𝑒𝑟𝑛𝑎𝑙 + 𝑃𝑓𝑙𝑢𝑖𝑑 Density of fluid, ρ and of manometer fluid, ρm Height of liquids are measured relative to the reference line Pressure on the left side: o 𝑃𝐴 = 𝜌𝑔𝑥 Pressure on the right side: (derivation of pressure difference from pressure balance) 𝑃𝐴 + 𝜌𝑔𝑥 = 𝑃𝐵 + 𝜌𝑔(𝑥 − ℎ) + 𝜌𝑚 𝑔ℎ o ∆𝑃 = 𝑃𝐴 − 𝑃𝐵 = (𝜌𝑥 − 𝜌)𝑔ℎ Two-fluid manometer o 2 manometer fluids o Fig 4 o Only has 1 liquid but can also be 2 liquids o Portrays how brakes work on vehicles Study on how formula were derived from principles Atmospheric pressure o Zero point for a relative pressure scale Perfect Vacuum o Zero point for absolute scale Vacuum o Pressure below atmospheric pressure o Region which the pressure is considerably lower than the atmospheric pressure o 𝑃𝑣𝑎𝑐𝑢𝑢𝑚 = 𝑃𝑎𝑡𝑚 − 𝑃𝑎𝑏𝑠 o As the vacuum value increases, the value of absolute pressure measured decreases Draft o In inches H2O o Pressure that is only slightly below atmospheric pressure o Not really noticeable but can be measured o Is also vacuum Absolute pressure: Patm Derive formula using pressure balance Pressure balance can also be applied not just only to U-tube manometer problems Fig 5 o 𝑃𝐴 + 𝜌𝑔𝑥 = 𝑃𝐵 + 𝜌𝑔𝑥 − 𝜌𝑔ℎ + 𝜌𝑚 𝑔ℎ Can also be used in hydraulics problem Vacuum 0 Vacuum is positive in Pabs and negative in Pg Gauge pressure is negative with vacuum Relative pressure: 0 Patm Vacuum Barometer o Used to measure pressure of atmosphere psig : gauge pressure in pounds per square inch solve pressure equivalent in other units : (P/ρ)/g (??) Average molecular weight of air: 28.84 solve density of air: 𝜌𝑎𝑖𝑟 = 𝑖𝑑𝑒𝑎𝑙 𝑔𝑎𝑠 𝑙𝑎𝑤 𝑃𝑉 = 𝑛𝑅𝑇 𝑚 𝑃𝑉 = 𝑅𝑇 𝑀𝑊 𝑚 𝑃(𝑀𝑊) = 𝑅𝑇 𝑣 𝑚 𝑃(𝑀𝑊) 𝜌= = 𝑣 𝑅𝑇 o P is absolute pressure in atm o T is in Kelvin o MW is in g/mol o R = 0.08206 L•atm/ mol•K Percent error o = o = |𝑡𝑟𝑢𝑒 −𝑚𝑒𝑎𝑠𝑢𝑟𝑒𝑑 𝑣𝑎𝑙𝑢𝑒| 𝑔 𝑔 𝑃𝐴 + 𝜌𝑜𝑖𝑙 ( ) 𝑥 = 𝜌𝑤𝑎𝑡𝑒𝑟 ( ) ℎ 𝑔𝑐 𝑔𝑐 ℎ= 𝑥 100% 𝑡𝑟𝑢𝑒 𝑣𝑎𝑙𝑢𝑒 𝑎𝑐𝑡𝑢𝑎𝑙−𝑒𝑥𝑝𝑒𝑐𝑡𝑒𝑑 𝑣𝑎𝑙𝑢𝑒 𝑥 𝑒𝑥𝑝𝑒𝑐𝑡𝑒𝑑 𝑣𝑎𝑙𝑢𝑒 100% Density is usually neglected for gases like air Ex: Solving z: 𝑔 𝑃𝐴 = 𝑃𝑎𝑖𝑟 + 𝜌𝑚 ( ) 𝑧 𝑔𝑐 𝑧= If Pg is used on the left then also use Pg on the right x was given -_Solving h: 𝑃𝐴 − 𝑃𝑎𝑖𝑟 𝑔 𝜌𝑜𝑖𝑙 (𝑔 ) 𝑐 𝑔 𝑃𝐴 + 𝜌𝑜𝑖𝑙 (𝑔 ) 𝑥 𝑐 𝑔 𝜌𝐻2𝑂 (𝑔 ) 𝑐 You should be constant if you use absolute or gauge pressure in pressure balance Keep track if you neglect atmospheric pressure in the calculations Recall that psig is without Patm Standard atmosphere vs. atmospheric pressure o Standard pressure is assumed to be in a standard gravitational field o Standard pressure is a fixed value o Standard pressure: 1 atm, 760 mmHg at 0˚C o Atmospheric pressure is a variable (it varies) o Atmospheric pressure is measured (usually from barometers) It’s easier to convert pressure units by relating to atmospheric pressure The nature of the instrument used to make the measurement, decides whether the pressure measured is relative or absolute Tank attached to a u-tubed manometer is below atmospheric pressure when the left leg attached to the tank is higher than the right leg For manometer with three fluids o o Solution process is similar to pressure balance o Pick a reference level for measuring pressure o If fluid 1 and 3 are gases and fluid 2 is Hg: Ignore the terms involving gases Density of gases are very small and can therefore be neglected 𝑃1 + 𝜌1 𝑑1 𝑔 = 𝑃2 o If fluid 1 and 3 are liquids and fluid 2 is immiscible Fluids 1 and 3 cannot be neglected 𝑃1 + 𝜌1 𝑑1 𝑔 = 𝑃2 + 𝜌2 𝑑2 𝑔 + 𝜌3 𝑑3 𝑔 Pressure drop o Experienced by flowing fluid when it passes through a restriction (i.e. orifice) Examples: o Pressure gauge on a tank of CO2 used to fill soda water bottles, connected to a barometer (absolute pressure) o Pressure difference across an orifice (pressure drops) Collab on 09-18-21 Ionic Compounds o Usually already in its empirical formula They do not consist discrete molecular units o Also called electrovalent compounds o Electrically neutral ionic compound Zero total charge Subscript of cation = anion and subscript of anion = cation o Metallic cation + nonmetallic anion Binary compounds o Formed from only two elements Ternary compounds o Consists of three elements Naming for Ionic Compounds o Before roman numerals: -ous Cation with fewer positive charges -ic Cation with more positive charges o Uses Roman Numerals for charges (called as the “Stock system”) It is much more specific Provides info of the actual charge of the cation o Roman numerals is the charge of the cation i.e. the subscript of the anion o Metallic cation + nonmetallic anion (-ide) Mercury I is a diatomic ion > Hg22+ Molecular Compounds o Contain discrete molecular units o Formed with nonmetallic elements o Usually binary compounds Naming Molecular Compounds o Uses Greek prefixes to signify number of atoms present Mono- : 1 Di- : 2 Tri- : 3 Tetra- :4 Penta- : 5 Hexa- : 6 Hepta- : 7 Octa- : 8 Nona- : 9 Deca- : 10 o Nonmetallic ion + nonmetallic ion (-ide) o The prefix mono- may be omitted for the first element o For oxides: the prefixes ending in “a” is omitted o Exceptions: Molecular compounds containing H B2H6 : diborane CH4 : methane SiH4 : silane NH3 : ammonia PH3 : phosphine H2O : water H2S : hydrogen sulfide o Usually straightforward Naming Acids o Acids: yield H+ ions when dissolved in water o In gaseous or pure liquid state: Hydrogen + nonmetallic anion o When dissolved in water: Hydro+ nonmetallic anion (-ic) + acid o Oxoacids: has H, O, and another element (the central element) + 1 O to “-ic” acid : “per…ic” acid - 1 O from “-ic” acid : “ous” acid - 2 O from “-ic” acid : “hypo…ous” acid o Oxoanions : anions of oxoacids All H are removed from “-ic” acid : ends with “ate” – all H from “-ous” acid : ends with “-ite” o Anions -H but not all H : indicate no. of H present Parent acids o Hydrofluoric acid, HF o Hydrochloric acid, HCl o Hydrobromic acid, HBr o Hydroiodic acid, HI o Hydrocyanic acid, HCN o Water, H2O o Hydrosulfuric acid, H2S o Hydroselenic acid, H2Se o Hydrotelluric acid, H2Te o Chloric acid, HClO3 o Bromic acid, HBrO3 o Iodic acid, HIO3 o Nitric acid, HNO3 o Acetic acid, HC2H3O2 o Carbonic acid, H2CO3 o Sulfuric acid, H2SO4 o Selenic acid, H2SeO4 o Telluric acid, H2TeO4 o Phosphoric acid, H3PO4 o Arsenic acid, H3AsO4 o Stibinc acid, H3SbO4 Naming Bases o Base: yields OH- ions when dissolved in water o Ion + hydroxide Writing chemical equations o Chemical equations: uses chemical symbols to show what happens in a reaction Shorthand expression for a chemical change/reaction Summarize reaction Display reacting substances Indicate amount of all reacting component substances Tells you the reactants and products involved Tells you the mole ratios of involved substances o 𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡𝑠 → 𝑝𝑟𝑜𝑑𝑢𝑐𝑡𝑠 o Includes additional info of physical states as subscripts (g) : gas (l) : liquid (s) : solid (aq) : aqueous environment o Arrow indicates direction of reaction (read as “yields”) o Above the arrow: Additional substance / factor supplied to the reaction Delta sign : +heat H2O : reactant is added to water; or substance reacted in the presence of water hv : light Oxidizing agents Solvents Catalysts, energy sources, environmental/reaction conditions o Below the arrow: Catalyst : alters speed of chemical reaction o Anything above and below the arrow is not consumed in the reaction o Upward arrow in products Evolution of a gas/gas bubbles In gaseous state o Downward arrow in products Formation of precipitate In solid state Balancing equations o Adjusting the coefficients so no. atoms of a substance in reactants is equal in its product o Law of conservation of mass o Subscripts cannot be changed or the identity of the substance will be altered Know how to predict products of chemical reactions Molecular Formula o Shows the exact number of atoms of each element Allotrope o A distinct form of an element o Example: Oxygen: oxygen gas (O2) and ozone (O3) Carbon: diamond and graphite Types of Chemical Reactions o Combination or Synthesis Reaction 𝐴 + 𝐵 → 𝐴𝐵 𝑚𝑒𝑡𝑎𝑙 + 𝑜𝑥𝑦𝑔𝑒𝑛 → 𝑚𝑒𝑡𝑎𝑙 𝑜𝑥𝑖𝑑𝑒 Solid + gas = gas basic 𝑛𝑜𝑛𝑚𝑒𝑡𝑎𝑙 + 𝑜𝑥𝑦𝑔𝑒𝑛 → 𝑛𝑜𝑛𝑚𝑒𝑡𝑎𝑙 𝑜𝑥𝑖𝑑𝑒 Solid/gas + gas = gas acidic 𝑚𝑒𝑡𝑎𝑙 + 𝑛𝑜𝑛𝑚𝑒𝑡𝑎𝑙 → 𝑠𝑎𝑙𝑡 Solid + gas/liquid = solid 𝑚𝑒𝑡𝑎𝑙 𝑜𝑥𝑖𝑑𝑒 + 𝑤𝑎𝑡𝑒𝑟 → 𝑚𝑒𝑡𝑎𝑙 ℎ𝑦𝑑𝑟𝑜𝑥𝑖𝑑𝑒 Solid + liquid = aq basic 𝑛𝑜𝑛𝑚𝑒𝑡𝑎𝑙 𝑜𝑥𝑖𝑑𝑒 + 𝑤𝑎𝑡𝑒𝑟 → 𝑜𝑥𝑦 − 𝑎𝑐𝑖𝑑 Gas/solid + liquid = aq Oxy-acid: also oxoacids/ ternary acid (HaXbOc) o Decomposition Reaction 𝐴𝐵 → 𝐴 + 𝐵 𝑠𝑜𝑚𝑒 𝑚𝑒𝑡𝑎𝑙 𝑜𝑥𝑖𝑑𝑒 → 𝑚𝑒𝑡𝑎𝑙 + 𝑂2(𝑔) 𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒𝑠 → 𝑚𝑒𝑡𝑎𝑙 𝑜𝑥𝑖𝑑𝑒 + 𝐶𝑂2(𝑔) 𝑏𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒𝑠 → 𝑚𝑒𝑡𝑎𝑙 𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 + 𝑤𝑎𝑡𝑒𝑟 + 𝐶𝑂2(𝑔) 𝑎𝑛𝑦𝑡ℎ𝑖𝑛𝑔 𝑤𝑖𝑡ℎ 𝑂 → 𝑠𝑢𝑏𝑠𝑡𝑎𝑛𝑐𝑒 + 𝑂2 Needs heat to separate O2 gas o Single Displacement Reaction 𝐴 + 𝐵𝐶 → 𝐵 + 𝐴𝐶 A is metal 𝐴 + 𝐵𝐶 → 𝐶 + 𝐵𝐴 A is halogen Requires activity/reactivity series 𝑚𝑒𝑡𝑎𝑙 + 𝑎𝑐𝑖𝑑 → ℎ𝑦𝑑𝑟𝑜𝑔𝑒𝑛 + 𝑠𝑎𝑙𝑡 𝑚𝑒𝑡𝑎𝑙 + 𝑤𝑎𝑡𝑒𝑟 → ℎ𝑦𝑑𝑟𝑜𝑔𝑒𝑛 + 𝑚𝑒𝑡𝑎𝑙 𝑜𝑥𝑖𝑑𝑒/ ℎ𝑦𝑑𝑟𝑜𝑥𝑖𝑑𝑒 𝑚𝑒𝑡𝑎𝑙 + 𝑠𝑎𝑙𝑡 → 𝑚𝑒𝑡𝑎𝑙 + 𝑠𝑎𝑙𝑡 ℎ𝑎𝑙𝑜𝑔𝑒𝑛 + ℎ𝑎𝑙𝑖𝑑𝑒 𝑠𝑎𝑙𝑡 → ℎ𝑎𝑙𝑜𝑔𝑒𝑛 + ℎ𝑎𝑙𝑖𝑑𝑒 𝑠𝑎𝑙𝑡 o Double Displacement or Metathesis Reaction 𝐴𝐵 + 𝐶𝐷 → 𝐴𝐷 + 𝐶𝐵 Exchange of +&- groups 𝑎𝑐𝑖𝑑 + 𝑏𝑎𝑠𝑒 → 𝑠𝑎𝑙𝑡 + 𝑤𝑎𝑡𝑒𝑟 Neutralization of an acid&base Formation of insoluble precipitate 𝑚𝑒𝑡𝑎𝑙 𝑜𝑥𝑖𝑑𝑒 + 𝑎𝑐𝑖𝑑 → 𝑠𝑎𝑙𝑡 + 𝑤𝑎𝑡𝑒𝑟 Formation of a gas Metathesis reactions include evolution of heat, formation of insoluble precipitate and production of gas bubbles in the product Anything that reacts with oxygen gas requires heat No reaction for metals and salts with metallic cation who has stronger activity/reactivity Activity/Reactivity Series o Likelihood to undergo single displacement o SPMGC don’t displace H Au, Pt, Ag, Hg, Cu o H o Double C NLT displace H from HA Co, Cd, Ni, Pb, Sn o CIZAM displace H from H2O(g) and HA Cr, Fe, Zn, Al, Mg o NaLiBaKCa displace H from H2O(l&g) and HA o For halogens, arrangement is based on electronegativity Diatomic Ions o H, Cl, Br, N, O, F, I Empirical Formula o Smallest number ratio o Shows the elements present in the smallest number ratio o The simplest Charges o Boron: ±3 o C : +4 o Si : ±4 Collab on 9/21/21 Stoichiometry o From Greek stoicheion, element, and metron, measure o Quantitative means of relating products to reactants Stoichiometric Coefficients o The relative amounts of moles of products and reactants Stoichiometric Ratios o The relative proportion of products and reactant o Used to relate amounts of substances from each other Reactants o Limiting Reactant, LR Theoretically first to be completely consumed Has the smallest maximum extent of reaction o Excess Reactant, ER Reactants that are not the limiting reactant Maximum extent of reaction o Quantity based on assuming complete reaction of each reactant o A straightforward way of determining which is the LR Solving for LR and ER o o 𝑛𝑓𝑒𝑒𝑑 𝑛𝑒𝑞𝑛 𝑛 𝑔𝑖𝑣𝑒𝑛 = 𝑛 𝑐𝑜𝑚𝑝𝑜𝑛 𝑒𝑞𝑛 𝑛𝑓𝑒𝑒𝑑 = 𝑐𝑜𝑚𝑝 𝑚𝑐𝑜𝑚𝑝 𝑀𝑊𝑐𝑜𝑚𝑝 𝑛𝑐𝑜𝑚𝑝 𝑜𝑛 𝑒𝑞𝑛 o Smallest ratio is the LR Conversion o Also termed as the degree of completion o Fraction of the reactant in the feed converted to products o In Himmelblau conversion and degree of completion is interchangeable, but in the SIM conversion is for all other reactants while degree of completion is only for the LR o %𝐶𝑜𝑛𝑣𝑒𝑟𝑠𝑖𝑜𝑛 = 𝑛𝑢𝑠𝑒𝑑𝑅 𝑛𝑓𝑒𝑒𝑑𝑅 × 100% Selectivity o Ratio of moles of desired product to undesired byproduct Yield o There is no universal definition for this, but there are three common o Yield based on feed: amount of desired product obtained over amount of LR fed o Yield based on reactant consumed: amount of desired product obtained over amount of LR consumed o Yield based on 100% conversion: amount of product obtained over theoretical amount of product based on LR fed o Third is dimensionless except for first two Yield and selectivity o Measure the degree to which desired reaction proceeds relative to competing undesirable reactions Exam this week (Saturday) o Stoichiometry and Material Balance Degree of Completion and Percent Yield o o Collab on 9/28/21 𝐷𝑜𝐶 = 𝑛𝐿𝑅 𝑟𝑒𝑎𝑐𝑡𝑒𝑑 𝑛𝐿𝑅 𝑓𝑒𝑒𝑑 Numerator: calculated from actual yield Denominator: calculated from theoretical yield o Value of degree of completion = value of percent yield o They only differ on perspective Degree of Completion focuses on reactant while percent yield focuses on products Selectivity o Ratio of desired product to undesired product o Measurement of finding of process to select which is either the first or second reaction o Comparison of 2 equation 𝑆= 𝑛 𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡 𝑐𝑜𝑛𝑣𝑒𝑟𝑡𝑒𝑑 𝑡𝑜 𝑑𝑒𝑠𝑖𝑟𝑒𝑑 𝑃 𝑛 𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡 𝑐𝑜𝑛𝑣𝑒𝑟𝑡𝑒𝑑 𝑡𝑜 𝑢𝑛𝑑𝑒𝑠𝑖𝑟𝑒𝑑 𝑃 Consecutive reactions o Reactions where the product becomes the reactant to another reaction and so on Material Balances o Application of law of conservation of mass Matter is neither created nor destroyed o Involves accounting for material o Always specify the system Unlike mass, volume is generally not conserved due to varying densities Terms in Material Balance o System A portion or the entire process itself that is taken for analysis Closed system: no material enters/leaves Open or flow system: material enters/leaves o System boundary A line (usually dotted) that encloses the system taken for analysis o Material Moles or any quantity conserved Components of material balance o Initial condition (input) o Final condition (output) o Generation Occur due to chemical reaction o Consumption Occur due to chemical reaction o Accumulation Can be negative Sum of all materials accumulated in the system over time interval Unit: mass/moles Unit can never be rate (mass/mole per unit time) Steady-state system or process o The amount or property of the material is invariant (does not change) Pseudo steady-state o Not entirely steady-state but is treated as such for convenience Quasi steady-state o Not entirely steady-state but is effectively behaving as such due to its slow rate of change Unsteady-state or transient process/model o The material in its initial condition is different from its final condition or material undergo changes over time Continuous Process o Material enters/leaves the system without interruption Batch Process o A closed process that treats a fixed amount of material over time o Material enters, material is processed, material is removed Semi-batch process o An open process where materials enter but never leave Difference equation: o 𝐴𝑐𝑐𝑢𝑚𝑢𝑙𝑎𝑡𝑖𝑜𝑛 = 𝑖𝑛 − 𝑜𝑢𝑡 + 𝑔𝑒𝑛 − 𝑐𝑜𝑛 Material Balance has assumptions: o The process are at steady-state Properties inside the system does not change Amount/ mass in system stays constant Implies accumulation=0 o No chemical reactions No generation and consumption Only happens when new chemical species are formed Implies gen&con=0 o Material Balance then is: 𝑖𝑛 − 𝑜𝑢𝑡 = 0 𝑖𝑛 = 𝑜𝑢𝑡 𝑚𝑎𝑠𝑠 𝑖𝑛 = 𝑚𝑎𝑠𝑠 𝑜𝑢𝑡 Chemical engineering operations focused on material balance o Chemical reactions o Fluid transport o Size reduction and enlargement o Heat generation and transport o Distillation o Evaporation o Gas absorption o Crystallization o Drying Box the overall material balance (OMB) to set the boundary Problems often should only use 1 component Each component that enters the system has its own component material balance For multiple component material balance, overall material balance may not be steady-state balances, since some other material accumulates or evaporates In this type of material balance we are only concerned with what goes in and out and disregard the process Always use linear equations for modelling If info is in ratio, cross multiply o Ex: X/Y = 0.2 will become X=0.2Y o Collab on 9/30/21 Exam o o o o o 2 h and 15 mins 15 mins for submission and no extension 3 questions 1 is stoichiometry 2 is two-stage material balance 3 is material balance with recycle stream Bypass Stream o Skips 1 or more stages o Some parts are fed to the process and some are left unprocessed o Used to control the composition of the final exit stream to obtain desired product with suitable proportions Recycle Stream o Increases efficiency of process o Gives higher yield of output o Can offer significant economic savings for high-volume processing systems o Usually accompanied with purge stream Purge Stream o Stream is bled off o Usually seen in reactive systems/processes involving reactions o Used to remove an accumulation of inerts or unwanted materials that might build up in the recycle stream o Usually placed with recycle streams, distillation columns Do Overall Material Balance (OMB) of whole system first then towards each subsystems Distillation Column o Unit operations that separates a mixture of 2 liquids with different boiling points Reflux o Some part of the distillate is returned to the distillation column o Increase the concentration of the low boiler (usually the desired product) Reflux/recycle ratio o Ratio of final product and recycle Benzene has lower boiling point than toluene Crystallization o Separation process for soluble solids o Lowering the solubility o Combination of evaporation (minimizes the solvent) and lowering the temperature Streams from the separator have the same properties K2CrO4 is nonvolatile For product with two states and different composition o Mass fraction of product (mass of stream)(mass fraction of component in the product) Multiple unit systems Operations o Mixer Combines two or more streams o Splitter Has 1 feed stream and produces two or more product streams with the same composition as the feed stream Key word is split, so stream is only “split” to different directions (still of the same composition) o Separator 1 or more stream of different composition entering and 1 or more streams still of different composition leaving Key word is separate, so there is separation of different species General rule: the basis you choose and the unit with which you start the analysis affect the degree of complexity of your calculations Distillation o Purify or separate alcohol in the beverage industry and hydrocarbons in the petroleum industry o Components of liquid mixture are separated by boiling Due to difference in vapor pressure Absorption o Occurs in absorption of oxygen from air Fermentation process, sewage treatment plant o Absorption of hydrogen gas for liquid hydrogenation of oil o A component is removed from a gas stream by treatment with a liquid Drying o of grain and other food is similar to drying of lumber, filtered precipitates, and rayon yarn o volatile liquids (usually water) are removed from solid materials Evaporation o of salt solutions (chemical) is similar to evaporation of sugar solutions (food) o evaporation of volatile solvent from nonvolatile solute Membrane Separation o Separation of solute from a fluid o Using diffusion through semipermeable membrane barrier Liquid-liquid extraction o Solute in liquid solution is removed by contacting with a relatively immiscible liquid solvent Adsorption o A component of a gas or liquid stream is removed and adsorbed by solid adsorbent Liquid-solid leaching o Dissolves and remove solute from a finely divided solid by treating it with a liquid that does the job Crystallization o Removal of solute from solution by precipitating the solute from solution Collab on 10/05/21 Final Exam o Will have about 5 problems o Unit conversion o Temperature o Pressure o Stoichiometry o Material Balance Recycle Bypass o o o o Multiphase systems Ideal gas equation On Tuesday, Oct.12 Review previous exam questions o Analytical chemistry : gravimetry Bypass Stream o Not everything is needed to be processed and is thus bypassed ṁ : indicates mass flow rate Collab on 10/08/21 Final Exam o 1:30-4:30 pm o Degrees of freedom o % humidity o 4b (multi-phase) is not included Gas Laws o Boyle’s Law At constant temperature, pressure is indirectly proportional to volume As pressure increases, volume decreases 𝑃𝑉 = 𝑘 𝑃1 𝑉1 = 𝑃2 𝑉2 o Charles’ Law At constant pressure, temperature is directly proportional to volume As temperature increases, volume increases o 𝑉 =𝑘 𝑇 𝑉1 𝑉 = 𝑇2 𝑇1 2 Gay-Lussac’s Law At constant volume, temperature is directly proportional to pressure 𝑃 =𝑘 𝑇 𝑃1 𝑃 = 2 𝑇1 𝑇2 o Combined gas Law o o o 𝑃𝑉 =𝑘 𝑇 𝑃1 𝑉1 𝑃 𝑉 = 2𝑇 2 𝑇1 2 Avogadro’s Law At constant temperature and pressure, number of moles is directly proportional to volume o o 𝑉 𝑛 o (𝑃 + 𝑎𝑛2 ) (𝑉 𝑉 − 𝑛𝑏) = 𝑅𝑇 o o =𝑘 Ideal gas Law 𝑃𝑉 𝑛𝑇 Where k is the ideal gas constant R 𝑃𝑉 𝑛𝑇 =𝑘 =𝑅 𝑃𝑉 = 𝑛𝑅𝑇 Dalton’s Law Of partial pressures 𝑃𝑡𝑜𝑡 = 𝑃1 + ⋯ 𝑃𝑛 𝑛1 𝑅𝑇 𝑉 𝑃1 = 𝑃1 = 𝑋1 𝑃𝑡𝑜𝑡 𝑛 𝑃1 = 𝑛 1 𝑃𝑡𝑜𝑡 𝑡𝑜𝑡 Amagat’s Law 𝑉𝑡𝑜𝑡 = 𝑉1 + ⋯ 𝑉𝑛 𝑉1 = 𝑋1 𝑉𝑡𝑜𝑡 𝑛 𝑉1 = 1 𝑉𝑡𝑜𝑡 𝑛𝑡𝑜𝑡 Good for mathematical estimates o Often used to estimate designs For designs which require exact parameters, use exact specific values for different parameters Real gas o Very specific o Has table of values o Has different type of equations Van der Waals equation o Alternative for ideal gas equation o Ideal gas equation + correction factors Ideal gas equation o Useful for alternatives of ideal gas behaviors o Used for rough estimates Ideal gas o Imaginary gas a and b are correction factors Correction factors are used since real gases deviate from properties of real gases o Each gases have different correction factors No real gas exactly obeys the gas laws Obeys ideal gas law o Lighter gas but with negligible deviations o Under ordinary circumstances o Vapor at low pressure and high temperature exudes behavior that approaches ideal gas Values of R 𝐿∙𝑎𝑡𝑚 𝑚𝑜𝑙∙𝐾 𝑓𝑡 3 ∙𝑝𝑠𝑖 𝑙𝑏𝑚𝑜𝑙∙°𝑅 𝑚3 ∙𝑃𝑎 𝑚𝑜𝑙∙𝐾 𝐿∙𝑡𝑜𝑟𝑟 𝑚𝑜𝑙∙𝐾 o 𝑅 = 0.08206 o 𝑅 = 10.73 o 𝑅 = 8.314 o 𝑅 = 62.36 When using the ideal gas equation, always state to assume ideal gas Relative scales are preferred since it is easy to relate and to imagine Always assume standard condition if pressure and temperature is not specified, 0˚C and 1 atm Formula weight of air sir preferred: 28.84 For specific gravity, write answers as o 𝑔𝑎𝑠 𝑎𝑡 𝑠𝑝𝑒𝑐𝑖𝑓𝑖𝑐 𝑇 𝑎𝑛𝑑 𝑃 𝑟𝑒𝑓𝑒𝑟𝑒𝑛𝑐𝑒 𝑔𝑎𝑠 𝑎𝑡 𝑠𝑝𝑒𝑐𝑖𝑓𝑖𝑐 𝑇 𝑎𝑛𝑑 𝑃 For density assuming ideal behavior, you do not need to derive just use: o [𝑣𝑎𝑙𝑢𝑒] 𝜌= 𝑃(𝑀𝑊) 𝑅𝑇 Partial Pressures: Dalton o Fictitious pressure exerted by a single component of gaseous mixture o Used by engineers … Be mindful if the percent given is in weight or mole Review for Analytical Chemistry, Gravimetry Analytical Chemistry is just advance stoichiometry Gravimetric methods: o Quantitative method o Based on determining the mass of a pure compound to which the analyte is chemically related The chemical species to be analyzed Precipitation gravimetry o Analyte is converted to sparingly soluble precipitate Precipitation gravimetry in a nutshell o Filter, wash, heat, weigh Washing is to free precipitate from impurities Heating is for it to convert to a product of known composition Types of reagents o Specific reagents Rare React only with a single chemical species o Selective reagents More common React with limited number of species Characteristics of ideal precipitating agent o Easily filtered and washed free of contaminants o Sufficiently low solubility So no significant loss of the analyte occurs o Unreactive With constituents of the atmostphere o Of known chemical composition Characteristics of favorable precipitates (ppt) for gravimetry o Large particle size Easy to filter and wash Usually purer than finer precipitates Factors that affect particle size of ppt o Ppt solubility o Temperature o Reactant concentration o Rate at which reactants are mixed Particle sizes o Colloidal precipitate 10-7 to 10-4 cm in diameter Show no tendency to settle from solution o o o Difficult to filter Crystalline precipitate At least 10-4 or greater in cm diameter Settles spontaneously Easily filtered Determined by which process/mechanism of precipitate predominates Nucleation: a minimum number of atoms, ion, or molecules join together to give a stable solid Formed on the surface of suspended solid contaminants Results to a large number of colloids Particle growth: growth of existing nuclei Results to small number of crystalline Determined using relative supersaturation Von Weimann eqn = 𝑄−𝑆 𝑆 Q is concentration of solute S is its equilibrium solubility When value is large, ppt is colloidal When value is small, ppt is crystalline High relative supersaturation: nucleation Low relative supersaturation: particle growth Ways to minimize supersaturation o Elevated temperatures (increase solubility) o Dilute solutions (minimize Q) o Slow addition of precipitating agent (good stirring) o Controlling pH (if solubility depends on pH) Supersaturated solutions o Unstable solution o Higher solute concentration Colloidal particles are usually coagulated first before filtered Ways to hasten coagulation o Heating o Stirring o Adding electrolyte to medium Adsorption: ions are retained on the surface of the solid; adhering to the surface o Primary adsorption layer: ions on surface; usually positive charged o Counter-ion layer: contains sufficient excess of negative ions; balances charge of particle’s surface o Electric double layer: imparts stability to colloidal suspension; between primary adsorption and counter-ion layer Peptization: reverting a coagulated colloid to its dispersed state Digestion: precipitate is heated in mother liquor o Mother liquor: solution from which the precipitate is formed o Results to denser mass o Improves coagulation Coprecipitation: normally soluble compounds that is carried out with the precipitate o Solubility of that compound is not yet reached, so it appeared as precipitate Types of coprecipitation: o Surface adsorption Soluble compound as surface contaminant Improved by digestion, washing the solution with volatile electrolyte, or reprecipitation (filtered solid is redissolved amd reprecipitated) o Mixed-crystal formation Contaminant ion replaces an ion in the lattice of a crystal Ion must have the same charge and sizes must differ by no more than 5% Countered with the use of a different precipitating agent or by separating the ion before the final precipitation step o Occlusion Foreign ions trapped in counter-ion layer within growing crystal o Mechanical entrapment Trap portion of solution in a pocket when crystals lie close together during growth o First two are equilibrium processes o The last two are from kinetics of crystal growth Countered by low rate of ppt formation, low supersaturation, and digestion Homogeneous precipitation o A precipitating agent is generated in a solution of the analyte o Better suited for analysis o Precipitating agent appears gradually and homogenously throughout the solution o Relative supersaturation is kept low o Results in increased crystal size and improved purity Weighing form: the compound formed after precipitate is heated Heating precipitate o Removes solvent and volatile species o Decompose solid and form compound of new composition Gravimetric calculations takeaways o Calculating percent component given mass sample and mass ppt = o 𝑚𝑝𝑝𝑡 (𝑀𝑊𝑝𝑝𝑡 )(𝑀𝑅)(𝑀𝑊𝑐𝑜𝑚𝑝 ) × 100% 𝑚𝑠𝑎𝑚𝑝𝑙𝑒 Calculating mass of component given mass of another component = 𝑚𝑜𝑡ℎ𝑒𝑟𝑐𝑜𝑚𝑝 × 𝑀𝑊𝑜𝑡ℎ𝑒𝑟𝑐𝑜𝑚𝑝 × 𝑛𝑐𝑜𝑚𝑝 × 𝑀𝑊𝑐𝑜𝑚𝑝 𝑛𝑜𝑡ℎ𝑒𝑟𝑐𝑐𝑜𝑚𝑝 o Molar ratio is just formula based, number of moles in component and other component are dependent of each other Ex: 1 mol of Pb3O4 is to 3 mol pf PbO2 Focus on central atom or main common component Determining which weighing form gives greatest mass of precipitate Calculate mass of common component/molecule/a tom from mass of each weighing form then compare values = 1 𝑔 𝑐𝑜𝑚𝑝 × 𝑀𝑊𝑐𝑜𝑚𝑝 × 𝑛𝑊𝐹 × 𝑀𝑊𝑊𝐹 𝑛𝑐𝑜𝑚𝑝 %𝑝𝑝𝑡 = 𝑚𝑠𝑎𝑚𝑝𝑙𝑒 ( )(𝑀𝑊𝑝𝑝𝑡 )(𝑀𝑅)(𝑀𝑊𝑐𝑜𝑚𝑝 ) 100% Insoluble Ions Except in Alkali metals, Ca, Sr, Ba, NH4+ Alkali metals, NH4+ Alkali metals, NH4+ Alkali metals, Ca, Sr, Ba, NH4+ Alkali metals 2- S CO32PO43OH O2 Normally soluble ions, but can be insoluble - Cl BrISO42 Insoluble with Ag+, Pb2+, Hg22+ Ag+, Pb2+, Hg22+ Ag+, Pb2+, Hg22+ Ba2+, Pb2+, Ca2+, Sr2+ Soluble Ions o Nitrates, NO3o Acetates, CH3COO o Chlorates, ClO3o Perchlorates, ClO4- o 𝑚𝑠𝑎𝑚𝑝𝑙𝑒 = o Highest yield will produce the greatest mass Determining minimum mass of the sample given %content of precipitate and mass of component Use lowest % and solve 𝑚𝑐𝑜𝑚𝑝 (𝑀𝑊𝑐𝑜𝑚𝑝 )(𝑀𝑅)(𝑀𝑊𝑝𝑝𝑡 ) %𝑝𝑝𝑡 𝑖𝑛 𝑠𝑎𝑚𝑝𝑙𝑒 Determining maximum precipitate mass given mass of component and %ppt Use largest % References Brown, L.S., & Holme, T.A. (2011). Chemistry for engineering students, 2nd ed. Cengage Learning. Brown, T.L., Lemay, H.E., Bursten, B.E., Murphy, C.J., & Woodward, P.M. (2012). Chemistry: The central science, 12th ed. Prentice Hall. Geankopolis, C.J. (1993). Transport process and unit operations, 3rd ed. Prentice-Hall. Himmelblau, D.M., & Riggs, J.B. (2012). Basic principles and calculations in chemical engineering, 8th ed. Pearson Education. Professor: Engr. Ramiro Amon Compiled by: F.D.