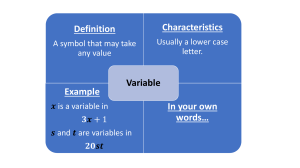
Blood gas coefficient The Blood Gas Partition Coefficient is also known as Ostwald coefficient for blood–gas. A partition coefficient is the ratio of the concentrations of a compound in one solvent to the concentration in another solvent at equilibrium. The blood/gas partition coefficient describes how the gas will partition itself between the two phases after equilibrium has been reached. For example: Enflurane has a blood/gas partition coefficient of 1.7. Therefore, if the gas is in equilibrium the concentration in blood will be 1.7 times higher than the concentration in the alveoli. Thus, it makes sense that a gas with a higher blood gas coefficient will require higher uptake of gas into the blood and induction will be slower. Key points: Higher partition coefficient = higher lipophilicity = higher potency = higher solubility High solubility = more anesthetic needs to be dissolved = slower onset MAC decreases as blood gas partition coefficient increases, generally speaking