Ovarian Cancer Case Study: Symptoms, Diagnosis & Treatment
advertisement

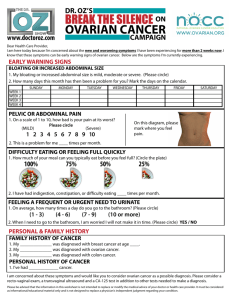
VICENTE G. GUADES, III PHARMACOLOGY LAB MD2A 03/29/2023 MODULE 10: OVARIAN CANCER CASE (HEMATOLOGY AND IMMUNOLOGY BLOCK) GUIDE QUESTIONS Clinical Questions: 1. What is ovarian cancer? - Ovarian cancer is when abnormal cells in the ovary begin to grow and divide in an uncontrolled way. They eventually form a growth (tumour). If not caught early, cancer cells gradually grow into the surrounding tissues. And may spread to other areas of the body. 2. How common is ovarian cancer? - Ovarian cancer is the leading cause of death in women diagnosed with gynecological cancers. It is also the fifth most frequent cause of death in women, in general. Most of the cases are diagnosed at an advanced stage, which leads to poor outcomes of this disease. - In 2020, there are approximately 21,750 new ovarian cancer cases, which comprises 1.2% of all cancer cases. The estimated number of deaths related to it are 13,940. The 5-year relative survival rate is expected to be 48.6%. Around 15.7% of the ovarian cancer cases are diagnosed at the local stage, and about 58% at the metastasized stage, where the 5-year survival dips down to 30.2% instead of 92.6% if detected at an early stage of local spread. An average incidence rate per 100,000, age-adjusted to the 2000 US standard population is 11.1 in 2012-2016. The incidence is highest in non-Hispanic whites (11.6 per 100,000), followed by American Indians and Alaska Natives (10.3 per 100,000), Hispanics (10.1 per 00,000), non-Hispanic blacks, and Asian and Pacific Islanders. Ninety percent of ovarian cancers are epithelial, with the serous subtype being the most common. Age-adjusted rates of new ovarian cancer cases are on a reducing trend based on statistical models of analysis. 3. What are the signs and symptoms of ovarian cancer? - Ovarian cancer may cause the following signs and symptoms: - Vaginal bleeding (particularly if you are past menopause), or discharge from your vagina that is not normal for you. - Pain or pressure in the pelvic area. - Abdominal or back pain. - Bloating. - Feeling full too quickly, or difficulty eating. - A change in your bathroom habits, such as more frequent or urgent need to urinate and/or constipation. 4. How is the diagnosis of ovarian cancer confirmed? - The presence of advanced ovarian cancer is often suspected on clinical grounds but can be confirmed only pathologically by removal of the ovaries or, when disease is advanced, by sampling tissue or ascitic fluid. - Current guidelines from the Society of Gynecologic Oncology and the American Society of Clinical Oncology recommend that the primary clinical evaluation for ovarian cancer include a computed tomography (CT) scan of the abdomen and pelvis with oral and intravenous contrast, and chest imaging (CT preferred) to evaluate the extent of disease and the feasibility of surgical resection. National Comprehensive Cancer Network guidelines recommend ultrasound and/or abdominal/pelvic CT or magnetic resonance imaging (MRI), as clinically indicated, and chest CT or - - - - - x-ray, as clinically indicated. Positron emission tomography (PET)/CT scan or MRI may be indicated for indeterminate lesions, if the results will alter management. MRI can increase the specificity of imaging evaluation in cases where the ultrasound appearance of the lesion is indeterminate. MRI is not definitive, however. On MRI, endometriotic cysts with enhanced mural nodules are a hallmark of ovarian cancer, but they may also be a feature of benign neoplasms and even inflammatory diseases. Large contrast-enhanced nodules on large endometriotic cysts in an elderly patient are more likely to indicate malignancy. When imaging studies demonstrate an adnexal mass, the decision whether to observe the patient with repeat imaging or to proceed to surgical evaluation must take into account not only the imaging characteristics but also the patient's medical history, physical examination results, and cancer antigen 125 (CA-125) level. Tumor markers such as CA-125 are not good discriminators of benign lesions from malignant lesions in premenopausal women but have better accuracy in postmenopausal women. In patients with diffuse carcinomatosis and gastrointestinal (GI) symptoms, a GI tract workup may be indicated, including one of the following: - Upper and/or lower endoscopy - Barium enema - Upper GI series Fine-needle aspiration (FNA) or percutaneous biopsy of an adnexal mass is not routinely recommended. In most cases, this approach may only serve to delay diagnosis and treatment of ovarian cancer. Instead, if a clinical suggestion of ovarian cancer is present, surgical evaluation for diagnosis and staging can be performed. An FNA, percutaneous biopsy, or diagnostic paracentesis should be performed in patients with diffuse carcinomatosis or ascites without an obvious ovarian mass, or in patients who will be treated with neoadjuvant chemotherapy. Approximately 50% of epithelial ovarian cancers have homologous recombination deficiency (HRD)—that is, deleterious mutations in homologous recombination DNA repair genes, such as BRCA1/2. Because HRD status is a biomarker for sensitivity to platinum and poly (ADP-ribose) polymerase (PARP) inhibitors, testing for BRCA mutations is indicated for patients with ovarian cancer. Patients without a germline deleterious BRCA mutation should be assessed for a somatic BRCA mutation. In those without a BRCA mutation, next-generation sequencing can identify other HRD features (eg, genomic instability). 5. What are the guidelines on ovarian cancer screening on the general population? - Ovarian cancer does not lend itself to screening because it has a relatively low prevalence within the general population and no proven precursor lesion exists that can be detected and treated to prevent the cancer from occurring. No approved screening method is available for ovarian cancer. - The U.S. Preventive Services Task Force (USPSTF) recommends against screening for ovarian cancer in the general population. The USPSTF found fair evidence that although screening with serum CA-125 level or transvaginal ultrasonography can detect ovarian cancer at an earlier stage, earlier detection is likely to have a small effect, at best, on mortality from ovarian cancer. In addition, because of the low prevalence of ovarian cancer and the invasive nature of diagnostic testing, the USPSTF concluded that the potential harms outweigh the potential benefits. - A randomized trial in a US population found that simultaneous screening with ultrasonography and CA-125 did not reduce ovarian cancer mortality, and evaluation of false-positive results was associated with complications. - The US Food & Drug Administration (FDA) recommends against the use of tests marketed for ovarian cancer screening. The National Cancer Institute (NCI) cites evidence of lack of mortality benefit with screening, and potential harms relating to false-positive test results. - - - Studies are trying to improve the accuracy of screening for early-stage ovarian cancer. Most are targeting perimenopausal or postmenopausal women or those with a family history of epithelial ovarian cancer. Many studies are using a combination of ultrasound, serum CA125 testing, and other tumor markers. Large prospective trials include the United Kingdom Collaborative Trial of Ovarian Cancer Screening, a European trial of ovarian cancer screening in 202,638 women; and the National Institutes of Health Prostatic, Lung, Colorectal and Ovarian (NIH-PLCO) cancer study. The primary outcome measure of the latter study is mortality from ovarian and fallopian tube cancer on 10-year follow-up. Primary analysis of data from the United Kingdom Collaborative Trial of Ovarian Cancer Screening found no significant difference in ovarian cancer mortality in women who underwent annual multimodal screening (MMS) with serum CA-125 interpreted with use of the risk of ovarian cancer algorithm, annual transvaginal ultrasound, or no screening. When prevalent cases were excluded, however, a significant mortality reduction with MMS was noted, with evidence of a mortality reduction in years 7-14. The authors conclude that "further follow-up is needed before firm conclusions can be reached on the efficacy and cost-effectiveness of ovarian cancer screening." Considerable interest has developed in the characterization of computer-analyzed protein patterns in the blood as a way of improving screening for ovarian cancer. Such methods are currently undergoing intensive research and clinical validation, and they may hold hope for the future. Lachance et al tested a nomogram for estimating the probability of ovarian cancer. The model had a sensitivity of 90% and a specificity of 73%, which may provide a further tool to aid in ensuring referral. In a study by van Nagell et al, asymptomatic women who underwent annual sonographic screening achieved increased detection of early stage ovarian cancer, with an increase in 5-year disease-specific survival. Pharmacology Questions: 1. List and identify the following the drugs stated above based on the ff: A. Generic name: Carboplatin - Carboplatin belongs to the group of medicines known as alkylating agents. Carboplatin interferes with the growth of cancer cells, which eventually are destroyed. - Indication: Advanced ovarian carcinoma; Small cell lung cancer - 2 most common side effects: Myelosuppression and Nausea and vomiting - Contraindications: Hypersensitivity to carboplatin or other platinum-containing compd (e.g. cisplatin). Patient w/ severe bone marrow depression or significant bleeding; bleeding tumours. Concomitant use w/ yellow fever vaccine. - Drug interactions: Carboplatin reacts w/ Al causing loss of potency and forming a precipitate, do not use needles, syringes, catheters, IV admin sets containing Al parts. Incompatible w/ amphotericin B cholesteryl sulfate complex. - Additive myelosuppressive effects w/ other myelosuppressive agents. Increased risk of nephrotoxicity and/or ototoxicity w/ aminoglycosides or diuretics. Excessive immunosuppression w/ risk of lymphoproliferation w/ ciclosporin. Increased risk of exacerbation of convulsions w/ phenytoin, fosphenytoin. Enhanced adverse/toxic effects of live attenuated vaccines. Potentially Fatal: Risk of generalised disease mortal w/ yellow fever vaccine. B. Generic name: Paclitaxel - Paclitaxel is an anti-cancer ("antineoplastic" or "cytotoxic") chemotherapy drug. Paclitaxel is classified as a "plant alkaloid," a "taxane" and an "antimicrotubule agent." Paclitaxel inhibits the microtubule structures within the cell. Microtubules are part of the cell's apparatus for dividing and replicating itself. Inhibition of these structures ultimately results in cell death. - Indication: Breast, lung, ovarian, gastroesophageal, prostate, bladder, and head and neck cancers 2 most common side effects: Bone marrow suppression (neutropenia); hypotension Contraindications: Solid tumours in patients with baseline neutrophil counts <1500 cells/mm3. Kaposi sarcoma in patients with baseline neutrophil <1000 cells/mm3 and serious uncontrolled infection. Severe hepatic impairment (as conventional solution). Lactation. Drug interactions: Increased plasma concentration and toxicity with CYP2C8 and CYP3A4 inhibitors (e.g. ketoconazole, erythromycin, fluoxetine, clopidogrel, cimetidine, ritonavir). Decreased plasma concentration and efficacy with CYP2C8 and CYP3A4 inducers (e.g. rifampicin, carbamazepine, phenytoin, efavirenz). C. Generic name: Bevacizumab - Bevacizumab works by interfering with the process of angiogenesis by targeting and inhibiting human vascular endothelial growth factor (VEGF). VEGF is a cytokine (a small protein released by cells that have specific effects on the behavior of cells) which when it interacts with its receptors in the cell leads to new blood vessel formation or angiogenesis. - Indication: Colorectal, breast, non-small cell lung, and renal cancer - 2 most common side effects: Hypertension, infusion reaction - Contraindications: Patient w/ CV disease, pre-existing HTN, CNS metastases, diabetes, history of arterial thromboembolism; at risk of thrombocytopenia, GI perforation and gall bladder perforation. Discontinue treatment if hypertensive crisis or hypertensive encephalopathy develops. Withhold treatment prior to elective surgery. Do not initiate treatment for at least 28 days after major surgery or until surgical wound is fully healed. Elderly >65 yr. Pregnancy and lactation. - Drug interactions: May increase risk of microangiopathic haemolytic anaemia w/ sunitinib. May increase risk of agranulocytosis and pancytopenia w/ dipyrone. May increase risk of agranulocytosis w/ clozapine. May increase risk of hand-foot skin reaction w/ sorafenib. May increase risk of osteonecrosis of the jaw w/ bisphosphonate derivatives. D. Generic name: Rucaparib - Indication: Maintenance treatment of adult women with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy. - 2 most common side effects: Black tarry stools, bloating or swelling of the face, arms, hands, lower legs, or feet. - Contraindications: None - Drug interactions: Using this medicine with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines. Warfarin 2. What is the overall approach to treatment of ovarian cancer? - Surgery is often the initial treatment of choice for ovarian cancer, provided patients are medically fit. Patients who are not candidates for optimal debulking should be considered for neoadjuvant chemotherapy followed by interval debulking surgery and further chemotherapy. Patients who are not fit for surgery may be given chemotherapy and considered for surgery later, or treated primarily with chemotherapy. - The aim of surgery is to confirm the diagnosis, define the extent of disease, and resect all visible tumor. The role of cytoreduction was demonstrated by Griffiths in 1975 and has been confirmed by many others. - Surgery should be used in conjunction with chemotherapy with a taxane and a platinum compound (eg, paclitaxel plus carboplatin). For more information on chemotherapy regimens, see Ovarian Cancer Treatment Protocols. - In patients with BCRA mutations, maintenance therapy with olaparib, with or without bevacizumab, after surgery and/or chemotherapy may significantly improve survival. In women who present with peritoneal carcinomatosis but without an obvious pelvic mass, an extensive search often fails to identify a primary tumor. These patients can be presumed to have ovarian carcinoma or primary peritoneal carcinoma and can be treated with cytoreductive surgery and platinum-based chemotherapy. References Green, A. E., & Sonoda, Y. (2022, November 17). Ovarian Cancer Treatment & Management: Approach Considerations, Choosing Appropriate Surgery, Surgical Staging. Medscape Reference. Retrieved March 30, 2023, from https://emedicine.medscape.com/article/255771-treatment Ovarian Cancer - StatPearls. (n.d.). NCBI. Retrieved March 30, 2023, from https://www.ncbi.nlm.nih.gov/books/NBK567760/ Rubraca (rucaparib) dosing, indications, interactions, adverse effects, and more. (n.d.). Medscape Reference. Retrieved March 30, 2023, from https://reference.medscape.com/drug/rubraca-rucaparib-1000121 Rucaparib (Oral Route) Side Effects. (2023, February 1). Mayo Clinic. Retrieved March 30, 2023, from https://www.mayoclinic.org/drugs-supplements/rucaparib-oral-route/side-effects/drg-20406019?p= 1 Selchick, F. (n.d.). Ovarian cancer: Causes, symptoms, and treatments. Medical News Today. Retrieved March 30, 2023, from https://www.medicalnewstoday.com/articles/159675#symptoms What Should I Know About Ovarian Cancer Screening? (n.d.). CDC. Retrieved March 30, 2023, from https://www.cdc.gov/cancer/ovarian/basic_info/screening.htm