1
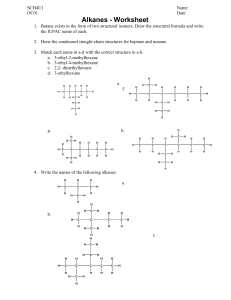
Alkanes
Homologous series
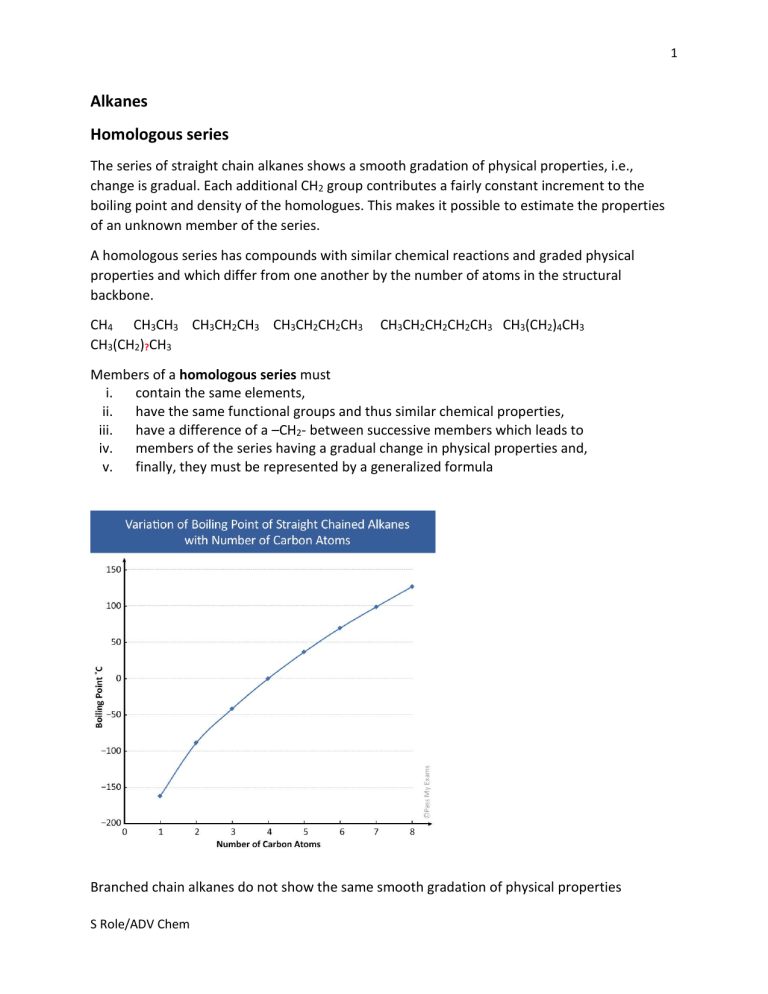
The series of straight chain alkanes shows a smooth gradation of physical properties, i.e.,
change is gradual. Each additional CH2 group contributes a fairly constant increment to the
boiling point and density of the homologues. This makes it possible to estimate the properties
of an unknown member of the series.
A homologous series has compounds with similar chemical reactions and graded physical
properties and which differ from one another by the number of atoms in the structural
backbone.
CH4 CH3CH3 CH3CH2CH3 CH3CH2CH2CH3
CH3(CH2)?CH3
CH3CH2CH2CH2CH3 CH3(CH2)4CH3
Members of a homologous series must
i. contain the same elements,
ii. have the same functional groups and thus similar chemical properties,
iii. have a difference of a –CH2- between successive members which leads to
iv. members of the series having a gradual change in physical properties and,
v. finally, they must be represented by a generalized formula
Branched chain alkanes do not show the same smooth gradation of physical properties
S Role/ADV Chem
2
Boiling points of branched alkanes:
Both hexane and 2,2-dimethylbutane have the same number of carbon atoms and hydrogen
atoms (C6H14). Explain why the boiling point of hexane is 68.7oC and the boiling point of 2,2dimethylbutane is 49.7oC.
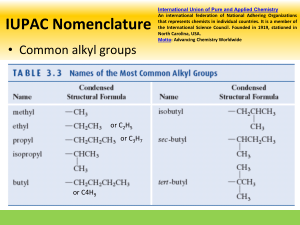
Nomenclature
Chemical nomenclature Booklet in VLE pg 11 (overview)
Nomenclature method, examples and questions in VLE.
General Formulae of (non-cyclic) alkanes CnH2n+2
Molecular Formulae
The molecular formula tells us how many atoms there are for each element in the
compound but not how the elements are bonded together. E.g., C4H10, C2H6O
Structural Formulae
A structural formula depicts how atoms are arranged and bonded together in a chemical
compound’s molecular formula.
Isomerism – Two categories
These two categories are Structural isomerism and Stereoisomerism
Structural isomerism – various types (Chain, [positional, functional group - discussed later])
S Role/ADV Chem
3
Chain (nuclear) isomerism
This is due to a different arrangement of the carbon skeleton of the molecules
Butane , methylpropane
Give the structural formulae of the isomers of pentane.
No 22 and 23 in Nomenclature worksheet.
Reactions of alkanes
General unreactivity
Alkanes are generally unreactive. They are either gases, ‘oily’ liquids or waxy solids. Insoluble in
water, but dissolve in organic solvents such as ethanol, ether and benzene.
But – Combustion reactions are vigorous!
Combustion of Alkanes (revision)
Alkanes burn in air to form CO2 and H2O. In low molecular weight alkanes, the flame is nonsmoky due to a low C-H ratio.
CH4 + 2O2 CO2 + 2H2O
Δ H = -890 kJmol-1
Gaseous alkanes burn explosively in air or oxygen
Incomplete combustion occurs in restricted amount of air, where C and toxic CO are also
formed.
Halogenation – Free radical substitution
Cl2 and Br2 react with alkanes in light/UV conditions
CH4 and Cl2 in sunlight – explosive reaction
No halogenation reaction in the dark.
Reaction with Cl2 or Br2 : In diffuse sunlight: a series of reactions take place. A photochemical
substitution reaction takes place where the hydrogen atoms in methane are replaced one at a
time by chlorine atoms. A mixture of organic products is produced. White fumes of HCl are
observed. This method is not ideal to obtain individual alkanes.
S Role/ADV Chem
4
CH4 + Cl2 CH3Cl + HCl
{ Several side products are produced}
(One way to reduce this problem is to use a much higher concentration of methane in
comparison to chloride. This reduces the chance of a chlorine radical running into a
chloromethane and starting the mechanism over again to form a dichloromethane. Through
this method of controlling product ratios one is able to have a relative amount of control over
the product.)
Mechanism of reaction to form chloromethane – Free radical mechanism; homolytic fission of
bonds
1. Initiation Phase - During this stage, the important radicals, which initiate the reaction
are formed in the presence of light or UV energy.
2. Propagation phase - The radicals are seen to react but more radicals are
produced so that the reaction may continue.
S Role/ADV Chem
5
3. Termination Phase - During termination stages, radicals come together to
produce ‘normal’ molecules. The radicals have been removed and the reaction
stops (or ‘terminates’).
Draw other side products that may form.
Cycloalkanes CnH2n
Examples of cycloalkanes
S Role/ADV Chem
6
Ring strain – comparing reactivities - [Sum of internal angles – 2(n-2)rt angles)
Cyclopropane (internal angle = 60o) - more reactive than propane
Cyclobutane (internal angle 90o) – more reactive than butane
Cyclopentane – little bond strain
Cyclohexane – relieve bond strain by puckering. Therefore, C6H12 is stable and unreactive as
hexane
Stereoisomerism (conformational, [geometrical, optical stereoisomerism – discussed later])
Conformational
The chair conformer of cyclohexane is completely free of strain.
The boat conformer of cyclohexane is completely free of
strain.
S Role/ADV Chem
7
Halogenation of cycloalkanes
The reactivity of cycloalkanes is similar to alkanes with the exception of the very small ones particularly cyclopropane.
In the dark, the ring breaks and an addition reaction occurs. The ring is broken because
cyclopropane suffers badly from ring strain. The bond angles in the ring are 60° rather than the
normal value of about 109.5° when the carbon makes four single bonds
In the presence of light, the above occurs together with substitution reactions.
Eg (one possibility) CH2BrCH2CH2Br + Br2 CHBr2CH2CH2Br + HBr
HYBRIDISATION in hydrocarbons
Hybridisation of atomic orbitals
This section explains the shape of the bonds and bond angles in alkanes (tetrahedral), alkenes
and carbonyl groups (planar) and alkynes (linear) in terms of hybridised sp3, sp2 and sp orbitals.
Types of bonds in covalent compounds
•
•
S
i
g
m bond (π ): sideways overlap of the lobes of unhybridised p-orbitals; only forms in
Pi
a presence of a sigma bond (electron density concentrated on either side of the
the
sigma bond) and where the two electrons lie in a region above and below the plane
b the molecule.
of
o
n
d
(σ) : an electron pair shared between 2 atoms by overlap of atomic orbitals on the
internuclear axis , so that electron density is concentrated between two nuclei. [the
two electrons are on imaginary line between the two nuclei]
S Role/ADV Chem
8
Discuss how a carbon atom C with an electronic structure of 1s2 2s2 2p2 forms four
identical, equally distributed bonds which give tetrahedral geometry in CH4 and CCl4?
Result - Methane – CH4 has four sp3 hybridised orbitals with which to bond with other atoms
In ethane – sp3 hybridisation. Alkanes adopt tetrahedral geometry.
The arrangement around each carbon atom is tetrahedral with approximately 109.50 bond
angles.
Same hybridisation occurs in other alkanes.
Read the following
https://www.chemguide.co.uk/basicorg/bonding/methane.html
S Role/ADV Chem
9
Ethene : uses sp2 hybridised orbitals and one un-hybridised p orbital ; Alkenes adopt planar
geometry around the double bond
In Ethyne – sp hybridised orbitals and two un-hybridised p orbitals
Alkynes adopts linear geometry around the triple bond.
S Role/ADV Chem