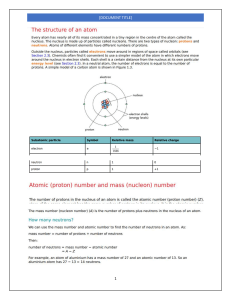
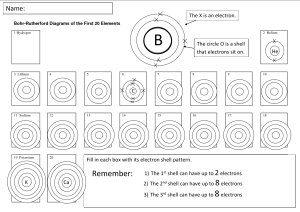
Elements 1 - 30 with Atomic Number and Symbol Atomic Number Symbol Element Name 1 H Hydrogen 2 He Helium 3 Li Lithium 4 Be Beryllium 5 B Boron 6 C Carbon 7 N Nitrogen 8 O Oxygen 9 F Fluorine 10 Ne Neon 11 Na Sodium 12 Mg Magnesium 13 Al Aluminum 14 Si Silicon 15 P Phosphorus 16 S Sulfur 17 Cl Chlorine 18 Ar Argon 19 K Potassium 20 Ca Calcium 21 Sc Scandium 22 Ti Titanium 23 V Vanadium 24 Cr Chromium 25 Mn Manganese 26 Fe Iron 27 Co Cobalt 28 Ni Nickel 29 Cu Copper 30 Zn Zinc The electron shells are often denoted by letters, and the first four shells are named K, L, M, and N. These letters correspond to the principal quantum numbers (n values) associated with each shell: K Shell (n=1): The innermost shell, closest to the nucleus. It can hold a maximum of 2 electrons. L Shell (n=2): The second shell, which is located further from the nucleus than the K shell. It can hold a maximum of 8 electrons. M Shell (n=3): The third shell, even farther from the nucleus. It can hold a maximum of 18 electrons. N Shell (n=4): The fourth shell, located beyond the first three. It can hold a maximum of 32 electrons. Element Valency H 1 He 0 Li 1 Be 2 B 3 C 4 N 3, 5 O -2 F -1 Ne 0 Na 1 Mg 2 Al 3 Si 4 P 3, 5 S -2 Cl -1 Ar 0 K 1 Ca 2 Sc 3 Ti 4 V 5 Cr 6 Mn 7 Fe 2, 3 Co 2 Ni 2 Cu 2 Zn 2