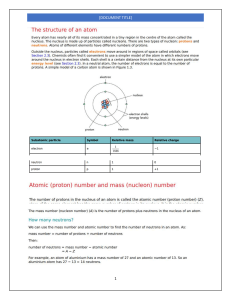
Science 9 Q2-Module3 Electronic Structure of Matter Ions: How They Are Formed? Lesson What I Need to Know Learning Competency: Explain how ions are formed; S9MT-IIe-f-16 Objectives: At the end of this module, you will be able to: 1. describe the general tendencies of atoms. 2. describe how positive and negative ions are formed. 3. classify ions through its composition, kind of charge and magnitude of charge. What’s In In the past lesson, you learned that the quantum mechanical model of the atom describes the atom as having a nucleus at the center. An electron is imagined to be cloud of negative charge having a certain geometrical shape. The electrons are arranged in principal or main energy levels that consist of one or more sublevels. The way in which electrons are distributed in the different orbitals around the nucleus of an atom is called electron configuration. Filling of electrons start from lower energy level to highest energy level. What’s New Look at the electron configuration of the elements in Group IA, IIA, IIIA, VIA and VIIA. Note that all elements have inner shells of s2p6 configuration except lithium which has only an s2 or helium-like inner shell. Group IA Example: Li - 1s2 2s1 Na Inner shell or (He) 2s1 -1s2 2s2 2p6 3s1 or (Ne) 3s1 Group IIA Example: Inner Shell 2 2 Be - 1s 2s or (He) 2s2 Mg - 1s2 2s2 2p6 3s2 or (Ne) 3s2 Group IIIA Example: Inner shell 2 2 1 B - 1s 2s 2p or (He) 2s22p1 2 2 6 2 1 Al - 1s 2s 2p 3s 3p or (Ne) 3s23p1 Figure 1.1Electron configuration of some elements in Group IA, IIA and IIIA The filled shell may be represented by the noble gas with a similar electron configuration. The noble gas symbol is followed by the valence shell symbol. Group IA atoms have electron configurations ending in an s1. This is in the valence shell of the atom. It contains only 1 electron, hence the number of electrons here indicates the group number IA. Group IIA elements have electron configurations ending in s 2, this is also in the valence shell of the atom. There are only two electrons here, hence the group IIA name. Group VIA Example: Inner shell O - 1s2 2s2 2p4 or (He) 2s2 2p4 S - 1s2 2s2 2p6 3s2 3p4 or (Ne) 3s2 3p4 Group VIIA Example: F - 1s2 2s2 2p5 or Cl - 1s2 2s2 2p6 3s2 3p5 or Inner shell (He) 2s2 2p5 (Ne) 3s2 3p5 Figure 1.2Electron configuration of some elements in Groups VIA, and VIIA An atom of a VIA element has 6 electrons in its outermost energy level. It tends to attract 2 electrons from another atom during chemical reaction to complete the 8 electrons. By so doing, it assumes the closed configuration of an inert gas. In general, outermost s electrons are loosely held by the nucleus. They are lost by the atom during chemical reactions. By so doing, it assumes the closed configuration of an inert gas and become very stable. Other elements tend to have this kind of stability. They are tend to acquire a noble gas configuration which has eight valence electrons through gaining or losing of electron(s). This is known as the Octet Rule. On the other hand, some elements whose outermost energy level is almost filled 6 to 7 electrons tend to share their outer electrons to other atoms to attain stable configuration. Metal elements tend to lose electrons to become stable while non-metal elements gain electrons to attain stability. Activity 1. Copy and fill out the table below. You may refer your periodic table. Element Atomic Number Electron configuration Ex. Sulfur Calcium Phosphorous Potassium 16 20 15 19 1s22s22p63s23p4 Give up valence electron or Attracts electron attracts electrons Inert gas it assumes (Ne) 3s23p4 What Is It Atoms have an equal number of protons and electrons and so do not have an overall charge. The nucleus of an atom is unchanged by ordinary chemical processes, but atom can readily gain or lose electrons. If electrons are given up (lose) or added (gain) to a neutral atom, a charged particle called an ion is formed. Ions have an unequal number of protons and electrons and so have an overall charge. How do Atoms form Positive ions? Positive ion is formed when an atom loses an electron to attain stability and has more protons than electrons and so has a positive overall charge. A (+) charge particle or (+) ion is called cation (pronounced cat-ion). The electron configuration of an atom shows how many electrons it must lose or gain to have a filled outer shell or to become stable. Atoms with a nearly empty outer shell, will lose electrons to obtain a full outer shell. Metal atoms, such as sodium, calcium and magnesium, form positive ions. Positive ions have a small ‘ + ’ symbol and a number by this to indicate how many electrons have been lost. This number is usually the same as the number of electrons in the atom’s outer shell. The net charge on an ion is represented by a superscript; +, 2+, 3+ mean a net charge resulting from the loss of one, two or three electrons respectively. Examples below show how sodium ion is formed: Sodium atom: atomic number =11 Sodium ion: 11 protons = +11 11 protons = +11 11 electrons = -11 10 electrons = -10 Total charge = 0 Total charge = +1 Dot and cross diagrams to represents ions of Sodium atom: + Loses 1 electron Na (barely full outer shell) Example 2: Magnesium atom: atomic number=12 12 protons = 12 electrons = Total charge = + 12 12 0 Na (full outer shell) Magnesium ion: 12 protons = 10 electrons = Total charge = + 12 10 + 2 - Dot and cross diagrams to represent ions of Magnesium atom: 2+ Loses 2 electrons Mg Mg ( barely full outer shell) (full outer shell) How do atoms form negative ions? Non-metals have high ionization energy and readily take in electron.When non-metal takes in an electron, there is an imbalance of charges making it negatively charge particle. An atom that gains an electron to attain stability is called a negative ion and has more electrons than protons and so has a negative overall charge. A (-) charge particle or negative (-) ion is called anion (pronounced an-ion). Non-metal atoms, such as chlorine, oxygen and nitrogen, form negative. Negative ions have a small ‘ _ ’ symbol and a number by this to indicate how many electrons have been gained to fill their outer shell. The net charge on an ion is represented by a superscript; -, 2-, 3- mean a net charge resulting from the gain of one, two or three electrons respectively. Example 1: How is a fluoride ion formed? Fluorine atom: atomic number = 9 +9 9 protons = -9 9 electrons = Total charge = 0 Fluoride ion: 9 protons = 10 electrons = Total charge = +9 -10 -1 Dot and cross diagrams to represent ions of Fluorine atom: - Gains 1 electron F (partially full outer shell) F (full outer shell) Example 2: How is a sulphide ion formed? Sulfur atom: atomic number =16 16 protons = 16 electrons = Total charge = +16 Sulfide ion: 16 protons = 18 electrons = Total charge = -16 0 +16 -18 -2 Dot and cross diagrams to represent ions of Sulfur atom: 2- Gains 2 electrons S (partially full outer shell) S (full outer shell) Try this: Draw dot and cross diagrams to represent ions of the following atom: a. Aluminum atom: atomic number=13 b. Oxygen atom: atomic number = 8 What’s More In this activity, you will classify ions through charges (cation or anions), composition (monoatomic or polyatomic), magnitude of charge (uni- or monovalent, divalent, trivalent, and so on) Classification of Ions and their Examples Classification Examples By Monoatomic (composed of only one atom) Fe2+, Li+, N3-, O2composition Polyatomic ion (composed of more than one SO42-, ClO3-, CO32atom) By kind of Cation (positive ion) Na+, Mg2+, Al3+ charge Anion (negative ion) Cl1-, O2-, IBy magnitude Monovalent (+1 or -1) Li+ , K+, OHof charge Divalent (+2 or -2) Ca2+, SO42-, Cu2+ Activity 2: Instruction: Fill in the table below. Write your answer in a separate sheet of paper. Classification Ions Ex. Mg2+ 1. NO32.H+ 3.P-3 4.CrO42- Composition (Mono atonic or polyatomic) monoatomic Charge (Cation or Anion) cation Magnitude of charge (Monovalent or Divalent) divalent What I Have Learned Instruction: See some ions inside the banner. Classify ions according to their similarities and differences by listing them in the appropriate table below: Ca2+ Cl- OH- Na+ ClO3CO32- IO3Al3+ Similarities: Cation & Monoatomic Differences: Anion & polyatomic Monovalent Divalent Trivalent What I Can Do In the mountains and on the hills, you feel rested. Why? It is because there is less dust in the air to consume the negative ions. By the seashores and near the waterfalls, you feel good. The energy of moving water generates a lot of negative ions. Instruction: Create a slogan to preserve and protect our mountains, seashores or waterfalls (just choose one 1). Use 1 short bond paper for your output. Consider the following Rubric in grading your Slogan: Category/Value Relevance/Concept 10 x 4= 40 pts. Artistic Presentation 10 x 4= 40 pts. Needs Improvement-3 Excellent-10 Good-7 Fair-5 The slogan shows relevance to the topic, completeness of the concept and clearly visualized. The slogan shows relevance to the topic, completeness of the concept, but the visualization somewhat unclear. The concept of the slogan shows partially relevance to the topic, and visualization of the concept is somewhat unclear. The concept of the slogan is erroneous or irrelevant. Artistic / colorful presentation of the slogan is clearly identified and visualized. Artistic / Colorful presentation of the slogan is identified, but it’s effect is in a somewhat unclear manner. Artistic / Colorful presentation of the slogan is partially identified, and is shown in an unclear manner. No Art / not Colorful presentation of the slogan Submission/Completion Used time well and submitted on 10 x 2= 20 pts. time. Used time well Did the output but Did the output but but not submitted did not appear submitted for on time. very interesting. compliance only. SCIENCE 9: QUARTER 2-MATTER MODULE 2: STUDENT’S ANSWER SHEET Name: ____________________ Grade: _____ Section: _________ Score: _____ (A) What I need to know Activity 1 Atomic Number Element Calcium 20 Phosphorous 15 Potassium 19 Electron configuration Give up valence electron or Attracts electron Inert gas it assumes (B) What Is It (write answers at the back) (C) What’s More Activity 2 Classification Ions Ex. Mg2+ Composition (Mono atonic or polyatomic) monoatomic Charge (Cation or Anion) cation Magnitude of charge (Monovalent or Divalent) divalent 1. NO32.H+ 3.P-3 4.CrO42- (D) What I have Learned Similarities: Cation & Monoatomic Anion & polyatomic Differences: (E) What I can do (use 1 short bond paper) Monovalent Divalent Trivalent