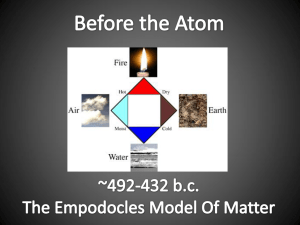
Chemistry Atomic Structure Dalton’s Atomic Theory In 1808, John Dalton came up with a series of postulates (assumptions) concerning the atom. These later became known as Dalton’s Atomic Theory. These are stated below: 1) 2) 3) 4) 5) Matter is made up of small particles called atoms Atoms are indestructible/indivisible Atoms of the same element are identical in mass and properties Atoms combine chemically in simple whole number ratios to form compounds Atoms can combine in more than one simple whole number ratio