Difference Between Electronic and Ionic Conduction Compare the Difference Between Similar Terms
advertisement

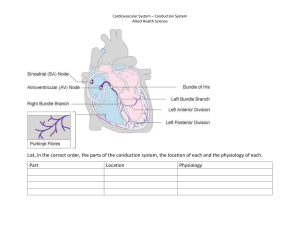
Compare the Difference Between Electronic and Ionic Conduction Difference Between Similar Terms April 17, 2020 Posted by Madhu (https://www.differencebetween.com/author/madhus/) 5 The key difference between electronic and ionic conduction is that electronic conduction is the movement of electrons (https://www.differencebetween.com/difference-between-ions-and-vs- electrons/) from one place to another, whereas ionic conduction is the movement of ions (https://www.differencebetween.com/difference-between-ions-and-vs-electrons/) from one place to another. The term conduction refers to the transfer of energy through a substance. Here, the energy (https://www.differencebetween.com/difference-between-energy-and-exergy/#Energy) can be transferred in different forms such as heat and electricity. Electronic conduction and ionic conduction are two forms of energy transferring methods which are categorized based on the medium of conduction. CONTENTS 1. Overview and Key Difference 2. What is Electronic Conduction 3. What is Ionic Conduction 4. Side by Side Comparison – Electronic vs Ionic Conduction in Tabular Form 5. Summary What is Electronic Conduction? Electronic conduction is the process of transferring energy in the form of an electric current (https://www.differencebetween.com/difference-between-conventional-current-and-vs-electric-current/). Here, the method of conduction is electron movement. However, any electron in any system cannot contribute to this conduction method. Electrons have to be in a free state in order to move from one place to another. The inner shell electrons of atoms cannot move. Another requirement is the presence of an electric field that can cause the movement of free electrons. Figure 01: Conduction of Electrons Electrons that are able to undergo conduction are called “conduction electrons”. These electrons are not firmly attached to any atom or a molecule. These free electrons can jump from the orbital of an atom to an orbital of an adjacent atom. However, as a whole, these electrons are bound to the conductor. The movement of electrons starts with the application of an electric field. The electric field gives the electrons a direction to move. What is Ionic Conduction? Ionic conduction is the process of transferring energy via the movement of ionic species. During ionic conduction, different ionic species move from one place to another place according to an ionic gradient. An ion is a charged species; it can be either positively charged or negatively charged. Positively charged ions move towards negatively charged places and vice versa. The tendency of a substance towards ionic conduction is measured as ionic conductivity. It is denoted by λ. Figure 02: A membrane cell used in the electrolysis of brine solution where ionic conduction occurs through the membrane at the middle to keep the ionic concentrations stable. Most often, we use the term ionic conduction regarding crystal lattices. Here, ionic conduction refers to the movement of ions from one defect to another in the crystal lattice. The process of conduction of ions is a mechanism of current where energy is passed from one place to another. Start Download (Free) PDF Hub Download What is the Difference Between Electronic and Ionic Conduction? Electronic conduction and ionic conduction are two forms of energy transferring methods which are categorized based on the medium of conduction. The key difference between electronic and ionic conduction is that electronic conduction is the movement of electrons from one place to another, whereas ionic conduction is the movement of ions from one place to another. Below is a summary table of the difference between electronic and ionic conduction. Summary – Electronic vs Ionic Conduction Electronic conduction and ionic conduction are two forms of energy transferring methods which are categorized based on the medium of conduction. The key difference between electronic and ionic conduction is that electronic conduction is the movement of electrons from one place to another, whereas ionic conduction is the movement of ions from one place to another. Reference: 1. “Metallic Conduction.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., Available here (https://www.britannica.com/science/metallic-conduction). 2. “Electrical Conduction.” ScienceDaily, ScienceDaily, Available here (https://www.sciencedaily.com/terms/electrical_conduction.htm). Image Courtesy: 1. “Conduction E vs DOS” By Tem5psu – Own work (CC BY-SA 4.0) (https://creativecommons.org/licenses/by-sa/4.0)via Commons Wikimedia (https://commons.wikimedia.org/w/index.php?curid=34981094) 2. “Chloralkali membrane” By Jkwchui – Based on * Bommaraju, Tilak V.; Orosz, Paul J.; Sokol, Elizabeth A.(2007). “Brine Electrolysis.” Electrochemistry Encyclopedia. Cleveland: Case Western Rsserve University.MSN Encarta. “Chloralkali Electrolysis.” Archived 2009-10-31 (CC BY-SA 3.0) (https://creativecommons.org/licenses/by-sa/3.0/deed.en) via Commons Wikimedia (https://commons.wikimedia.org/w/index.php?curid=17868243) Sömntabletten alla pratar om Sleepmore 5 Related Posts: Difference Between Isolated System and Closed System Difference Between Work and Heat Difference Between Pyrolysis and Gasification (https://www.differe (https://www.differe Difference Between Homogeneous and Heterogeneous Equilibrium (https://www.differe ncebetween.com/di ncebetween.com/di ncebetween.com/di fference-between- fference-between(https://www.differe fference-between- work-and-vs-heat/) pyrolysis-andncebetween.com/di isolated-systemgasification/) fference-betweenand-vs-closedhomogeneous-andsystem/) heterogeneousequilibrium/) Difference Between Fusion and Solidification (https://www.differe ncebetween.com/di fference-betweenfusion-andsolidification/) About the Author: Madhu Madhu is a graduate in Biological Sciences with BSc (Honours) Degree and currently persuing a Masters Degree in Industrial and Environmental Chemistry. With a mind rooted firmly to basic principals of chemistry and passion for ever evolving field of industrial chemistry, she is keenly interested to be a true companion for those who seek knowledge in the subject of chemistry. HOME (HTTPS://WWW.DIFFERENCEBETWEEN.COM/) VACANCIES (HTTPS://WWW.DIFFERENCEBETWEEN.COM/VACANCIES/) ABOUT (HTTPS://WWW.DIFFERENCEBETWEEN.COM/ABOUT/) REQUEST ARTICLE (HTTPS://WWW.DIFFERENCEBETWEEN.COM/REQUEST/) CONTACT US (HTTPS://WWW.DIFFERENCEBETWEEN.COM/CONTACT-US/) Copyright © 2010-2018 Difference Between (https://www.differencebetween.com). All rights reserved. Terms of Use (https://www.differencebetween.com/about/terms-of-use/) and Privacy Policy: Legal (https://www.differencebetween.com/about/legal/).