40 Characteristic Studies on Adsorption of Methylene Blue Dye Using low cost natural adsorbent
advertisement

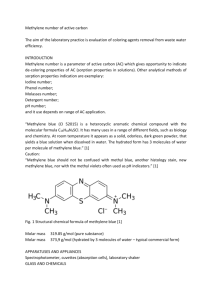
SPECIALUSIS UGDYMAS / SPECIAL EDUCATION 2022 1 (43) ___________________________________________________________________________________________________________ Characteristic Studies on Adsorption of Methylene Blue Dye Using Natural Low-Cost Adsorbent Ravi Vital Kandisa and Narayana Saibaba KV* Department of Biotechnology, GITAM School of Technology (GST), GITAM (Deemed to be University) Visakhapatnam-530045, (Andhra Pradesh) India *Corresponding author: skvn@gitam.edu, vittubiotech@gmail.com ABSTRACT In the present research,Vigna Trilobata pod was used as an adsorbent material for the removal of Methylene blue dye from an aqueous solution.This study isaimed to examine the characterization studies of adsorbent. Adsorbent surface and morphological characteristics, functional groups and adsorbent nature was examined by Scanning Electron Microscopy (SEM), Fourier-transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD) and Transmission Electron Microscopy (TEM). In this study, the porous nature, along with the surface size and shape of Vigna Trilobata pod confirmed by all the above four characterization methods. The prepared sample was tested for Methylene blue (MB) dye adsorption capacity. Key Words: Adsorption studies, Characteristic studies, Scanning Electron Microscopy, Transmission Electron Microscopy INTRODUCTION In the present days government has taken a serious initiative to reduce the pollution resulting from industrial activities, especially from textile industries. Therefore, there is a necessity to exploit effluents and reduce their effect on the environment (Taleb F et al. 2020)1. Methylene blue is a synthetic dye,used extensively in printing, chemical and textile industries which leads to impurethe natural water sources, causes harm to human and animal health as well as the environment. Instead of other techniques for the removal of dyes from wastewater such as ion exchange, chemical oxidation, membrane process, coagulation, and biodegradation, adsorption method has the most advantages, like the ease of operation and flexibility where it is necessary to identify novel low cost and naturally 9057 SPECIALUSIS UGDYMAS / SPECIAL EDUCATION 2022 1 (43) ___________________________________________________________________________________________________________ available adsorbents. It was reported in the available literature thatcomposites and activated carbons werethe most commonly used adsorbents for adsorption of Methylene blue dye which possess a great variety of modified surface groupswhich are helping in water purification (Alcaraz L et al., 2018, Costa-Marrero Y, et al. 2020 and Belhachemi M and Addoun F, 2011)2, 3, 4 . From the available literature, there is no such study that shows the utilization of Vigna Trilobata podas an adsorbent for the removal of dyes from an aqueous solution released by textile industries.The adsorbent, Vigna Trilobata pod is one of the naturally available adsorbents used for the removal of Methylene blue dye in the current study. In addition to our earlier adsorption studies (Kandisa RV et al. 2018, Narayana Saibaba KV and Kandisa RV, 2019 and Kandisa RV et al., 2021), we have also conducted a study on the characterization of Vigna Trilobata pod by using Fourier Transform Infrared radiation (FTIR), Scanning Electron Microscopy (SEM), X-Ray diffraction (XRD) and Transmission Electron Microscopy (TEM) analysis methods for the uptake of Methylene blue dye to report the adsorption capacity of the adsorbate. The obtained results from SEM and TEM studies help in identifying the morphology of the adsorbent by using adsorbate. FTIR techniquewas used to analyze the functional groups present in the adsorbent and XRD analysis was used to observe the nature of the adsorbent taken for the dye uptake and evaluated the suitability for the adsorption study5, 6, 7. EXPERIMENTAL PROCEDURE Sample Preparation: Adsorbate was prepared by adding 1.0 gram of Methylene blue dye powder to 1 liter of water. The solution was thoroughly mixed with a glass stirrer for uniform mixing and further diluted to various concentrations. In this experiment, adsorbent used was collected from natural resources. A naturally available plant source named Vigna Trilobata podwas collected from Marturu village located in Visakhapatnam district. The collected pod was cleaned and washed under the running tap water. Then the pure and cleaned Vigna Trilobata podextract was grinded and sieved to perform characterizationstudies. Characterization Studies: Scanning Electron Microscope was used to characterize the surface and morphology of the used adsorbent before and after Methylene blue dye adsorption. The sample was prepared by cleaning with acetone to make it free from foreign particles then fixed by using glutaraldehydeand dehydrate using ethanol; finally,gold coated prepared sample was mounted under the SEM chamber for analysis. 9058 SPECIALUSIS UGDYMAS / SPECIAL EDUCATION 2022 1 (43) ___________________________________________________________________________________________________________ Digitalized images were captured at various magnifications of 0.5 µm, 5µm, 10µm, 50µm, and 100µm and at an energy potential of 20kV with a working distance between 8.8 mm to 9.0 mm8. Fourier Transformation Infrared Spectroscopy (FT-IR)analysis was used to identify the functional groups within the range of 4000-400 cm-1. The sample was analyzed by using the KBR pellet method which helps to measure the powdered samples and for the detection of different functional groups. A low concentration of samples is required to obtain clear pellets and it should be between 0.2-1%. A finely grounded powdered sample should be taken to avoid adsorption band distortions and helps to obtain the standard spectrum. The samplewas subjectedto contact withan infra-red beam for FTIR analysis, the IR beam will absorb and transmitted through a sample to display a number of peaks at various wavelengths with respect to functional groups9-10. X-Ray Diffraction (XRD) patterns of the adsorbent analysis were carried out at room temperature by using ARL™ EQUINOX 100 X-ray Diffractometer instrument. XRD analysis was used to identify the molecular structure of the adsorbent. The sample was placed on an X-Ray diffractometer and was subjected to X-Ray diffractionwith a temperature range of 20-50֠C operated at a voltage of 20kV9, 11-13. Vigna Trilobata pod was also characterized by using Transmission Electron Microscope (TEM) - JEM1400Flash instrument. The sample was prepared and subjected to place on the specimen holder to conduct the experiment.TEM analysis plays a crucial role in the identification of the shape, morphology and size distribution of the sample in the adsorbent before and after adsorption of Methylene blue dye with an accelerated voltage of 200kV12, 14, 15. RESULTS AND DISCUSSION Scanning Electron Microscope (SEM): SEM images of the used adsorbent Vigna Trilobata pod fromthe Figure.1 (a) and 1 (b) were shown below. We can see the clear structure of the adsorbent at various magnifications. High-resolution images were captured at 140x, 950x, 1600x and 3700x magnificationsand observed the presence of a large number of micropores with a rough and irregular surface on the surface of the adsorbent before Methylene blue adsorption under the SEM model (JSM-7900F). From the figures,It indicates that the presence of micropores within the structure of Vigna Trilobata pod plays an important role as active adsorption sites during the removal of Methylene blue dye molecules and the pore size is within the range of 5µm to 100µm.The structure of the adsorbent after dye adsorption is smooth and the surface is 9059 SPECIALUSIS UGDYMAS / SPECIAL EDUCATION 2022 1 (43) ___________________________________________________________________________________________________________ completely filled with adsorbate leading to higher adsorption of the adsorbentresulting in successful adsorption of Methylene blue dye ontoVigna Trilobata pod. Fig. 1. (a). SEM images of Vigna Trilobata pod before adsorption at various resolutions 9060 SPECIALUSIS UGDYMAS / SPECIAL EDUCATION 2022 1 (43) ___________________________________________________________________________________________________________ Fig. 1 (b). SEM images of Vigna Trilobata pod after Methylene blue dye adsorption at various resolutions Fourier transform infrared spectroscopy (FTIR): FTIR spectra were analyzed before and after adsorption by using an FTIR spectrophotometer as shown in the below figures 2 (a) and 2 (b). It can be observed that obtained four peaks indicated the presence of different functional groups on the surface of the adsorbent. FTIR spectra from Fig 2(a) suggested the presence of powdered cellulose asthe main component.From fig. 2 (b) A Broad peak was observed at 3296.35cm-1 which represents the presence of the O-H group and a narrow peak observed at 1022.27cm-1 indicates the presence of a strong C-H stretching bond. The presence of C=C stretch and C-H stretch is ascertained from peaks ata wavelength of 1602.85cm-1 and 2918.30cm-1.It was observed (fig.2 (a)) that there are minimal changes in the spectral range of Methylene blue dye, which may concludethat there is no effect on functional groups present in the chosen sample of Vigna Trilobata podbefore and after adsorption. 9061 SPECIALUSIS UGDYMAS / SPECIAL EDUCATION 2022 1 (43) ___________________________________________________________________________________________________________ 2 (a): FTIR spectrum of Methylene blue dye before and after adsorption on to Vigna Trilobata pod 2 (b): FTIR spectrum of Vigna Trilobata pod after adsorption of Methylene blue dye 9062 SPECIALUSIS UGDYMAS / SPECIAL EDUCATION 2022 1 (43) ___________________________________________________________________________________________________________ X-Ray Diffraction (XRD) Analysis: A sample of Vigna Trilobata pod was taken and characterized by using X-raydiffractometer (Model: ARL™ EQUINOX 100) as shown inFigure 3. Results showed that obtained peaks at 2θ corresponding to the lattice planes 012, 110, 111, 013, 014, 114, 122 and 220 indicate the crystal phases of Methylene blue dye and the diffraction pattern shows a shift to 29A° which indicates the correlation of ions in dye molecules. From the obtained diffractogram, it was observed that the spectra exhibit the same degree of Crystallinity which results in the occurrence of the same peak positions before and after adsorption of the Methylene blue dye and confirms that the adsorbent used in the current study is witha crystalline structure and amorphous in nature14, 15. Fig3. XRD pattern of adsorbent Samples before dye adsorption Transmission Electron Microscope (TEM): Morphological distribution was further analyzed by using TEM analysis as observed in fig. 4 as it is one of the important tools for the characterization of the particles to reveal their size and shape. TEM analysis was conducted before and after Methylene blue dye adsorption at various magnifications.The resulting TEM micrographs were obtained in the form of high-resolution TEM images of the given sample recorded.TEM results were evaluated and it reveals that the average particle size is within the range of 15-35nm and most of the particles are spherical in shape with a narrow size and well-defined limited aggregation which confirms the porous nature and Crystallinity of the adsorbent. Also, it was observed that TEM results are correlating well with results obtained from SEM and XRD analysis. 9063 SPECIALUSIS UGDYMAS / SPECIAL EDUCATION 2022 1 (43) ___________________________________________________________________________________________________________ Fig.4. TEM images of Methylene blue dye on Vigna Trilobata pod before and after adsorption Conclusion: Vigna Trilobata pod sample was prepared and applied for various characterization studies like SEM, FTIR, XRD and TEM analysis for the removal of Methylene blue dye from an aqueous solution. The sample was prepared by using the KBR pellet method which can be useful for clear identification of functional groups by using FTIR analysisto confirm the presence of four different functional groups as mentioned above. Porus nature of the material was confirmed by SEM analysis, which also illustrates the surface and morphological characteristics of the adsorbent. The high Crystallinityand nature of the adsorbent were well defined by XRD and TEM analysis. By using TEM analysis, the average particle size was determined as 15nm. These characteristic studies reveal that the used adsorbent exhibits high crystallinity, spherical in shape, has good porosity, and amorphous in nature which confirms that it can be considered an efficient adsorbent for the removal of Methylene blue dye from the aqueous solution. Hence it can be suggested that Vigna Trilobata pod can be used for the dye removal process in the treatment of textile industry wastewater to eliminate the synthetic pollutants which cause severe harm to the environment. 9064 SPECIALUSIS UGDYMAS / SPECIAL EDUCATION 2022 1 (43) ___________________________________________________________________________________________________________ References 1. Taleb F, Ammar M, Mosbah MB, Salem RB, Moussaoui Y. Chemical modification of lignin derived from spent coffee grounds for Methylene blue adsorption. Scientific Reports. 2020 Jul 6;10(1):1-3. 2. Alcaraz L, Lopez Fernandez A, García-Díaz I, López FA. Preparation and characterization of activated carbons from winemaking wastes and their adsorption of methylene blue. Adsorption Science & Technology. 2018 Jul;36(5-6):1331-51. 3. Costa-Marrero Y, De Andrade MB, Ellena J, Duque-Rodríguez J, Farías T, Autié-Castro G. Zeolite/ZnO composites based on a Cuban natural clinoptilolite and preliminary evaluation in Methylene blue adsorption. Materials Research Express. 2020 Jan 20;7(1):015066. 4. Belhachemi M, Addoun F. Comparative adsorption isotherms and modeling of Methylene blue onto activated carbons. Applied water science. 2011 Dec;1 (3):111-7. 5. Kandisa RV, KV NS. Kinetic Studies on Adsorption of Methylene blue Using Natural Low Cost Adsorbent. Journal of Industrial Pollution Control. 2018; 34 (2):2054-. 6. KV NS, Kandisa RV. ADSORPTION ISOTHERM STUDIES ON METHYLENE BLUE DYE REMOVAL USING NATURALLY AVAILABLE BIOSORBENT. environmental protection.;3:4. 7. Kandisa RV, KV NS, Shaik KB, Gopinadh R. STUDIES ON EFFECT OF ADSORPTION PARAMETERS FOR THE METHYLENE BLUE DYE REMOVAL BY USING LOW-COST ADSORBENT. 8. Mosoarca G, Popa S, Vancea C, Boran S. Optimization, Equilibrium and Kinetic Modeling of Methylene blue Removal from Aqueous Solutions Using Dry Bean Pods Husks Powder. Materials. 2021 Jan;14(19):5673. 9. Gani A, Faisal M. Evaluation of liquid smoke-activated palm kernel shells biochar for cadmium adsorption. Rasayan J Chem. 2020;13:1451-7. 10. Lingeswari UD, Vimala T. ADSORPTION STUDY ON REMOVAL OF REACTIVE BLUE 21 AND REACTIVE RED 180 FROM AQUEOUS MEDIUM USING POLYANILINE CuCl2 IN THE PRESENCE OF UV LIGHT. ShahidiHub International Journal of Business, Economics & Development Studies. 2020 Aug 6;1(1):11-20. 11. Nath SK, Bhattacharyya KG, Das M. Adsorptive removal of fluoride from water using bamboo dust and its modified forms: Kinetics and isotherm study. 9065 SPECIALUSIS UGDYMAS / SPECIAL EDUCATION 2022 1 (43) ___________________________________________________________________________________________________________ 12. AllokoKj, Ekou T, Ekou L, Lafon O. Adsorption of lead on a natural clay from the agboville region (côted’ivoire) and activated clay with hydrochloric acid. Modeling by linear isotherms of Langmuir and Freundlich. 13. Jawad AH, Abdulhameed AS, Mastuli MS. Acid-factionalized biomass material for Methylene blue dye removal: a comprehensive adsorption and mechanism study. Journal of Taibah University for Science. 2020 Jan 1;14(1):305-13. 14. Ganapuram BR, Alle M, Dadigala R, Dasari A, Maragoni V, Guttena V. Catalytic reduction of Methylene blue and Congo red dyes using green synthesized gold nanoparticles capped by salmaliamalabarica gum. International Nano Letters. 2015 Dec;5(4):215-22. 15. Abebe B, HC AM, Zerefa E, Abdisa E. Porous PVA/Zn–Fe–Mn oxide nanocomposites: Methylene blue dye adsorption studies. Materials Research Express. 2020 Jun 3;7(6):065002. 16. Jaramillo-Fierro X, González S, Montesdeoca-Mendoza F, Medina F. Structuring of zntio3/tio2 adsorbents for the removal of methylene blue, using zeolite precursor clays as natural additives. Nanomaterials. 2021 Apr;11(4):898. 17. Mosoarca G, Popa S, Vancea C, Boran S. Optimization, Equilibrium and Kinetic Modeling of Methylene Blue Removal from Aqueous Solutions Using Dry Bean Pods Husks Powder. Materials. 2021 Jan;14(19):5673. 18. Thabede PM, Shooto ND, Naidoo EB. Removal of methylene blue dye and lead ions from aqueous solution using activated carbon from black cumin seeds. South African Journal of chemical engineering. 2020 Sep 23;33(1):39-50. 19. Jawad AH, Abdulhameed AS, Mastuli MS. Acid-factionalized biomass material for methylene blue dye removal: a comprehensive adsorption and mechanism study. Journal of Taibah University for Science. 2020 Jan 1;14(1):305-13. 20. Utsev JT, Iwar RT, Ifyalem KJ. Adsorption of methylene blue from aqueous solution onto delonix regia pod activated carbon: batch equilibrium isotherm, kinetic and thermodynamic studies. Agric. waste. 2020;4(5):18. 21. Shooto ND, Nkutha CS, Guilande NR, Naidoo EB. Pristine and modified mucuna beans adsorptive studies of toxic lead ions and methylene blue dye from aqueous solution. South African journal of chemical engineering. 2020 Jan 1;31:33-43. 9066 SPECIALUSIS UGDYMAS / SPECIAL EDUCATION 2022 1 (43) ___________________________________________________________________________________________________________ 22. Kahsay MH, Belachew N, Tadesse A, Basavaiah K. Magnetite nanoparticle decorated reduced graphene oxide for adsorptive removal of crystal violet and antifungal activities. RSC Advances. 2020;10(57):34916-27. 23. Obayomi KS, Oluwadiya AE, Lau SY, Dada AO, Akubuo-Casmir D, Adelani-Akande TA, Bari AF, Temidayo SO, Rahman MM. Biosynthesis of Tithonia diversifolia leaf mediated Zinc Oxide Nanoparticles loaded with flamboyant pods (Delonix regia) for the treatment of Methylene Blue Wastewater. Arabian Journal of Chemistry. 2021 Oct 1;14(10):103363. 24. Kahsay MH, Belachew N, Tadesse A, Basavaiah K. Magnetite nanoparticle decorated reduced graphene oxide for adsorptive removal of crystal violet and antifungal activities. RSC Advances. 2020;10(57):34916-27. 25. Joshi S, Shrestha RG, Pradhananga RR, Ariga K, Shrestha LK. High Surface Area Nanoporous Activated Carbons Materials from Areca catechu Nut with Excellent Iodine and Methylene Blue Adsorption. C. 2022 Mar;8(1):2. 26. Ali AF, Kovo AS, Adetunji SA. Methylene blue and brilliant green dyes removal from aqueous solution using agricultural wastes activated carbon. Journal of Encapsulation and Adsorption Sciences. 2017 Jun 12;7(2):95-107. 27. Babalola BM, Babalola AO, Akintayo CO, Lawal OS, Abimbade SF, Oseghe EO, Akinola LS, Ayanda OS. Adsorption and desorption studies of Delonix regia pods and leaves: removal and recovery of Ni (II) and Cu (II) ions from aqueous solution. Drinking Water Engineering and Science. 2020 Jul 17;13(2):15-27. 28. Shokry H, Elkady M, Hamad H. Nano activated carbon from industrial mine coal as adsorbents for removal of dye from simulated textile wastewater: Operational parameters and mechanism study. Journal of Materials Research and Technology. 2019 Sep 1;8(5):4477-88. 29. Manjari G, Saran S, Arun T, Rao AV, Devipriya SP. Catalytic and recyclability properties of phytogenic copper oxide nanoparticles derived from Aglaia elaeagnoidea flower extract. Journal of Saudi Chemical Society. 2017 Jul 1;21(5):610-8. 30. Khafri HZ, Ghaedi M, Asfaram A, Safarpoor M. Synthesis and characterization of ZnS: Ni-NPs loaded on AC derived from apple tree wood and their applicability for the ultrasound assisted comparative adsorption of cationic dyes based on the experimental design. Ultrasonics Sonochemistry. 2017 Sep 1;38:371-80. 9067