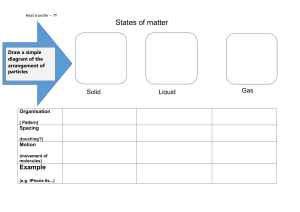
Name__________________________________ Date_______ Chemistry Pre Test True or False– An atom is stable only because its charge is equal to zero. What is an element? Group 17 metals form what charge Ion? Electrons are found where in an atom? For an ionic bond to form ______ must be transferred. (choose a subatomic particle) My charge is negative Does Na prefer to give away electrons or gain them? How many e-? My charge is ZERO What is the mass in amu of a proton? Neutrons are found where in an atom? What is the mass in amu of a neutron? What has to happen to for an atom to become an ion? The horizontal rows in the periodic table are called? How does an Ionic bond form? What has to happen for an atom to become an isotope? How do we calculate the number of electrons in an atom? True or false-atoms can have a positive charge and be stable. Group 16 metals form what charge Ion? What happens to the e- in an atom when energy is added to it in the form of light or heat. List three examples of a chemical reaction __________ ______________ Atomic number is equal to what? How do we calculate the number of neutrons in an atom? ____________ Name__________________________________ Date_______ Chemistry Pre Test What is the mass in amu of an electron? What is the formula to calculate neutrons on the periodic table? Opposite charges _________ Like charges _____________ Group 18 metals form what charge Ion? Separation using boiling points is called. Group 15 metals form what charge Ion? Phase changes are always what type of change? List three main phases of matter _____________ ______________ ____________ Most elements are ___________ Protons are found where in an atom? Atoms need this to be stable like noble gases, according to what rule? True or false-Ions can have a negative charge and be stable. The vertical columns in the periodic table. Group 1 metals like K form what charged Ion? How many valence electrons does Carbon have? True or false-Ions can have a positive charge and be stable. Group 18 metals form what charge Ion? What did we say in class is the reason that elements react for the most part in nature? Why do atoms expand usually when energy is added to them? What happens when an atom loses energy and goes from the “excited state” back to ground state? Name__________________________________ Date_______ Chemistry Pre Test How many valence electrons does Ar have? For this part put a less than < or greater than > to explain states of matter Distance between molecules(particles) solid ____ Distance between molecules(particles)liquid Distance between molecules(particles) Liquid ____ Distance between molecules(particles)Gas Distance between molecules(particles) solid ____ Distance between molecules(particles)Gas Distance between molecules(particles) solid ____ Distance between molecules(particles)liquid Speed of molecules(particles) solid ____ Speed of molecules(particles)Gas Speed of molecules(particles) liquid ____ Speed of molecules(particles)Gas Speed of molecules(particles) solid ____ Speed of molecules(particles)Liquid Deeper Thinking How many Li+ Ions would join with an Oxygen Ion if they were to react? Explain why you believe what you believe. How many F ions would join with Be ions for them both to stop reacting and be stable? Explain what happens to particle speed as you heat up and then cool down elements(don’t forget phase changes) Make a Sketch Draw a picture of how this quiz made you feel below: