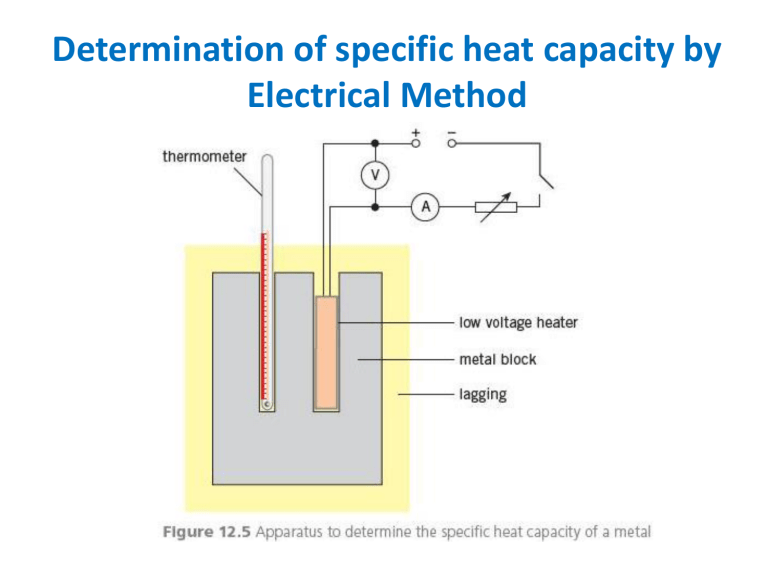
Determination of specific heat capacity by Electrical Method Determination of specific heat capacity of liquid by continuous flow Method Electrical energy supplied (E1) = V1I1t1 = m1c(θ2 - θ1 ) + H Electrical energy supplied (E2) = V2I2t2 = m2c(θ2 - θ1) + H Specific heat capacity of the liquid (c) = [E2 – E1]/(m2 – m1)(θ2 - θ1) Determination of Specific Latent Heat of Fusion of ice by Electrical Method (M-m)x L = Ivt Where M is mass of ice melted when heater is switched on in t seconds m is mass of ice melted before the heater is switched on in t seconds (M-m) is the mass of ice melted due Determination of Specific Latent Heat of Vaporisation of water by Electrical Method Numericals 1. A kettle is rated as 2.3 kW. A mass of 750 g of water at 20 °C is poured into the kettle. When the kettle is switched on, it takes 2.0 minutes for the water to start boiling. In a further 7.0 minutes, one half of the mass of water is boiled away. (a) Estimate, for this water, (i) the specific heat capacity, specific heat capacity. [4600 Jkg-1K-1] (ii) the specific latent heat of vaporisation [5] [2.6*106 Jkg-1] (b) State one assumption made in your calculations, and explain whether this will lead to an overestimation or an underestimation of the value for the specific latent heat. [2] 1. A jeweller wishes to harden a sample of pure gold by mixing it with some silver so that the mixture contains 5.0% silver by weight. The jeweller melts some pure gold and then adds the correct weight of silver. The initial temperature of the silver is 27 °C. Use the data given below to calculate the initial temperature of the pure gold so that the final mixture is at the melting point of pure gold. [1483 K] 1. A mass of 24 g of ice at –15 °C is taken from a freezer and placed in a beaker containing 200 g of water at 28 °C. Data for ice and for water are given below (i) Calculate the quantity of thermal energy required to convert the ice at – 15 °C to water at 0 °C. [3] [756+7920 = 8700 J] (ii) Assuming that the beaker has negligible mass, calculate the final temperature of the water in the beaker [160C] 1. Define specific latent heat of fusion.Use the kinetic theory of matter to explain why melting requires energy but there is no change in temperature. [2+3=5] a. A block of ice at 0 °C has a hollow in its top surface, as illustrated in Fig. A mass of 160 g of water at 100 °C is poured into the hollow. The water has specific heat capacity 4.20 kJ kg–1 K–1. Some of the ice melts and the final mass of water in the hollow is 365 g. [328 kJ kg–1 ] a. Assuming no heat gain from the atmosphere, calculate a value, in kJ kg–1, for the specific latent heat of fusion of ice. [3] b. In practice, heat is gained from the atmosphere during the experiment. This means that your answer to (i) is not the correct value for the specific latent heat. State and explain whether your value in (i) is greater or smaller than the correct value [2] Temperature and thermometer Temperature is average K.E. of molecules. It measure the degree of hotness of a body. Measure by an instrument called thermometer In order to measure temperature quantitatively some sort of numerical scale must be defined. Two temperature scales Fahrenheit and Celsius are used for general purpose and Kelvin scale for the scientific purpose. Temperature Scales Operation of all types of thermometers depends on some properties of matter that changes with temperature called thermometric properties. These changes can be used to measure the temperature. (e.g. length, resistance, pressure, voltage etc). The values of the property such as length are measured at 2 fixed temperatures the freezing point and a boiling point of water at standard atmospheric pressure. the interval between the 2 fixed temperatures is divided into a number of subintervals (empirical scale). The Celsius and Kelvin scale has 100 units between these points, while Fahrenheit scale has 180 units. This is known as calibration of thermometer. Common Temperature Scale Three common types – Celsius scale (oC) – Fahrenheit scale (oF) – Kelvin Scale (K) Establish the Relationship between three temperature scales Example 1. If C = 30 oC then F = 84 oF 2. What is the temperature for which Celsius and Fahrenheit thermometer shows the same reading? Common Temperature Scale Different type of thermometers 1. Liquid-in-glass thermometer 2. Resistance thermometer 3. Thermocouple thermometer 4. Constant Volume gas thermometer 5. Pyrometer Liquid-in-Glass Thermometer Liquid-in-glass thermometer 1. mercury-in-glass 2. alcohol-in-glass – Why not use water ??? • A relatively large bulb at the lower portion of the thermometer hold the major portion of the liquid, which expands when heated and rise in capillary tube upon which appropriate scale marking are fixed. The size of the capillary depends on the size of the sensing bulb, the liquid, and the desired temperature range for the thermometer. • At the top of the capillary tube another bulb is placed to provide a safety feature in case the temperature range of the thermometer is accidentally exceeded. Alcohol range -115oC to 78oC Mercury range -39oC to 357oC Operation of liquid-in-glass thermometer • Operate when the bulb is exposed to the environment whose temperature is to be measured. • A rise in temperature causes the liquid to expand in the bulb and rise in the capillary, thereby indicating the temperature. • It is important to note that the expansion registered by the thermometer is the difference between the expansion of the liquid and the expansion of the glass Finding Temperature Value of ( X 100 ) and ( X 0 ) of the temperature measuring property are found at steam point and at ice point.( X 100 X 0 ) is called fundamental interval of the scale. If ( X ) is the value of property at unknown temperature θ in ⁰C is given by X X 0 100 X 100 X 0 Mercury-in-glass thermometer Advantages – Expands evenly on heating – Responds quickly to temperature – A high boiling point, so used in hot places – It does not wet Disadvantages – Poisonous – Expensive – A high freezing point, so not used in cold places Alcohol-in-glass thermometer Advantages – Expands about six times of mercury – Expand evenly on heating – A low freezing point, so used in very cold places – It is safe – It is cheap Disadvantages – It is dyed – It wet the tube – It does not respond quickly with temperature – A low boiling point, so not used in hot places Clinical thermometer A specialized mercury in glass thermometer to take body temperature A constriction to prevent mercury from falling range a few degrees above and below 37oC Liquid in glass Thermometer Inaccuracies arise in thermometer from Advantages 1. Non uniformity of the bore of the capillary tube Simple, Cheap, Portable Direct reading, Everyday 2. The gradual change in zero use, Clinical use and owing to the bulb shrinking weather recording for number of years after manufacture 3. The mercury in the stem Disadvantages not being at the same Not very accurate temperature as that in the Small range (-39 to 500) bulb Questions 1. Length of mercury column at 0oC is 12 cm length of mercury column at 100oC is 22 cm. What is the temperature if the length of the column is 16 cm? 2. Length of mercury column at 37oC is 12.0 cm length of mercury column at 100oC is 26.1 cm. What is the length of the mercury column at temperature 0oC ? Resistance Temperature Detector (RTD) Quite accurate method of temperature measurement. Consists of some type of resistive element which is exposed to the temperature to be measured. The temperature is indicated through a measurement of the change in resistance of the element. Several types of materials may be used such as platinum, nickel, iron(alloy), copper and tungsten. Among them platinum is preferred Platinum Resistance Thermometer Why Platinum ? 1. It has high melting point 2. It is chemically stable R R0 100 R100 R0 3. Its resistance changes uniformly over the wide range of temperature Platinum Resistance Thermometer Advantages 1. Wide range (-200 to 1200) 2. Very accurate 3. Best for small steady temperature difference 4. Zero of this thermometer does not change because the resistance is always same at the same temperature Disadvantages 1. Unsuitable for rapidly changing temperature due to large heat capacity and low thermal conductivity of the container. 2. A small amount of impurities in platinum can produce great error in the temperature 3. Is not direct reading type Thermocouple When two metals are joined end to end to form a junction & these junction are kept at different temperature , a small emf is produced & current flows through the metals. The emf is called thermo emf & the effect is called thermoelectric or Seebeck effect. The pair of dissimilar metals forming the junction is called thermocouple which is the sensing junction. The most common electrical method of temperature measurement uses the thermocouple. Thermocouple, contd… • The magnitude of the thermo emf depends on the types of metals used for the wires and amount of temperature difference between the junction. Thermoelectric series (Seebeck series) • Sb,Fe,Cd,Zn,Ag,Au,Pb,Mn,Cu,Pt,Co,Ni,Bi Thermocouple Thermometer Advantages Disadvantages 1. Is not so accurate as 1. Wide range (-250 to 1500) platinum resistance 2. Fairly accurate thermometer for 3. Cheap and can easily be measuring temperature constructed below 1000 oC 4. Suitable for rapidly changing 2. Different thermocouple are temperature because to be used for different junction is small so it ranges. absorbs very small heat 3. It is difficult to maintained 5. Robust (Strong) and cold junction at a constant compact temperature. Constant-Volume Gas Thermometer • The physical property used in this device is the pressure variation with temperature of a fixed-volume gas. • The volume of the gas in the flask is kept constant by raising or lowering the reservoir B to keep the mercury level at A constant 28 Constant Volume gas Thermometer • Advantages • Disadvantages 1. Wide range (-270 to 1500) oC 2. Very accurate and sensitive 3. Cheap and can easily be constructed 4. Used as a standard to calibrate other practical type 1. It is bulky 2. Slow to respond 3. Not direct reading Absolute Zero • The thermometer readings are virtually independent of the gas in the flask. • If the lines for various gases are extended, the pressure is always zero when the temperature is – 273.15o C • This temperature is called absolute zero 30 Kelvin Temperature Scale The Kelvin temperature scale is now based on two new fixed points. Adopted in 1954 by the International Committee on Weights and Measures. One point is absolute zero and the other point is the triple point of water. Triple point of water This is the single Temperature and pressure at which ice, water, and water vapor can coexist in thermal equilibrium. The triple point of water occurs at 0.01o C and 4.58 mm of mercury .This temperature was set to be 273.16K on the Kelvin temperature scale. The Kelvin is defined as 1/273.16 of the temperature of the triple point of water 31 Thermistor Semiconductor device that has a negative temperature coefficient of resistance, in contrast to the positive coefficient displayed by most metals. NTC means, the resistance decreases as the temperature rises. Resistance at 25oC for typical commercial units ranges from 100 to over 100M. Very sensitive device and consistent performance within 0.01oC however it is highly nonlinear behavior. Since the resistance of the Thermistor is very high, the error due to resistance is small. In addition, the high resistance of the thermistor means the smaller current required for measurement. Thermistor resistance decreases very nonlinearly with increasing temperature. Suppose we take two resistance readings and average them. 250 Kohms 50 Kohms Avg = 150 Kohms Average resistance gives correct average temperature? No!! Must convert to temperature before averaging. Thermistor-based probes can contain electronics to give a linear voltage output with temperature. Pyrometers • • • • Pyrometer is used: To measure temperature of a very hot body such as are encountered in furnaces. Where thermometers cannot brought into contact or Where hot bodies are moving Measurements done by measuring energy radiated by a hot body or by comparison of colour Pyrometers



