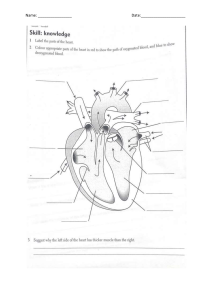
Foundation 3 CVS Arterial Blood Gas (ABG) A test that measures the levels of oxygen and carbon dioxide in the blood, as well as the blood's pH, which helps assess lung and kidney function and the body's overall acid-base balance. Arterial blood pressure The pressure exerted by the blood against the walls of arteries, primarily measured as systolic (pressure during heart contraction) and diastolic (pressure during heart relaxation) values, typically reported in millimeters of mercury (mm Hg). Atrial fibrillation A common heart rhythm disorder characterized by irregular and often rapid heartbeat originating in the atria, leading to inefficient pumping of blood into the ventricles. Autonomic nervous system A part of the nervous system responsible for involuntary functions like heart rate, digestion, and respiratory rate, divided into the sympathetic (fight or flight) and parasympathetic (rest and digest) branches. Aorta The largest artery in the body that originates from the left ventricle of the heart and carries oxygenated blood to the rest of the body. Arterio-venous Relating to the connection or communication between an artery and a vein. Atrioventricular node A small cluster of cells in the heart that acts as a natural electrical relay station, delaying the electrical signal between the atria and ventricles, allowing the ventricles to fill before contracting. Atrioventricular valve Valves located between the atria and ventricles of the heart; the tricuspid valve separates the right atrium and right ventricle, and the bicuspid (mitral) valve separates the left atrium and left ventricle. Blood pressure The force of blood against the walls of arteries, with systolic pressure (during heartbeats) and diastolic pressure (between heartbeats); usually expressed as systolic/diastolic (e.g., 120/80 mm Hg). Beats per minute A measure of heart rate, indicating the number of times the heart contracts (beats) in one minute. Cerebral blood flow The rate at which blood is delivered to the brain, crucial for maintaining brain function and supplying oxygen and nutrients to brain cells. Capillary hydrostatic pressure The pressure exerted by blood within capillaries, which forces fluid out of capillaries and into the surrounding tissues. Central nervous system The part of the nervous system that includes the brain and spinal cord, responsible for processing sensory information, thoughts, and motor commands. Cardiac output The volume of blood pumped by the heart in one minute, calculated as the heart rate multiplied by the stroke volume. Capillary oncotic pressure The osmotic pressure generated by proteins (mainly albumin) in the blood, which helps to draw fluid back into capillaries Cardiopulmonary pressure Pressure within the heart and lungs, typically referring to the pressures measured during medical procedures or tests. Central venous pressure The pressure in the vena cava that returns blood to the right atrium of the heart; it reflects the filling pressure of the right side of the heart. Diastole The phase of the cardiac cycle when the heart muscle relaxes and fills with blood. Diastolic pressure The lower number in a blood pressure reading, representing the pressure in arteries when the heart is at rest (during diastole). Deep vein thrombosis A condition where a blood clot forms in a deep vein, most commonly in the legs, which can be a serious medical condition if the clot breaks free and travels to the lungs. Electrocardiogram A test that records the electrical activity of the heart to evaluate its rhythm and detect any abnormalities. Echocardiogram A diagnostic test that uses ultrasound waves to create images of the heart's structure and function. End diastolic pressure 1. The pressure in the ventricles at the end of the diastolic filling phase, just before the next contraction. End diastolic volume The volume of blood in the ventricles at the end of diastole, just before contraction begins. Ejection fraction The volume of blood in the ventricles at the end of diastole, just before contraction begins. End systolic volume The volume of blood remaining in the ventricles at the end of systole (after contraction). Heart rate The number of times the heart beats in one minute, usually expressed as beats per minute (bpm). Inferior vena cava The large vein that carries deoxygenated blood from the lower half of the body to the right atrium of the heart. Left atrium One of the heart's four chambers that receives oxygenated blood from the lungs and pumps it into the left ventricle. Left atrial pressure The pressure in the left atrium of the heart, which reflects the filling pressure of the left ventricle. Left ventricle The heart's main pumping chamber that receives oxygen-rich blood from the left atrium and pumps it into the aorta to be distributed throughout the body. Mean artrial pressure The average pressure in the arteries during one cardiac cycle, calculated as onethird of the systolic pressure plus two-thirds of the diastolic pressure. Mean arterial blood pressure Synonymous with mean arterial pressure (MAP), representing the average pressure in the arteries. Myocardial infarction Also known as a heart attack, it occurs when blood flow to a part of the heart muscle is blocked, leading to tissue damage or cell death. Pulse pressure The difference between systolic and diastolic blood pressure, reflecting the force generated by the heartbeat. Pulmonary vascular resistance The resistance or opposition to blood flow in the pulmonary circulation, important for maintaining blood flow to the lungs. Right atrium One of the heart's four chambers that receives deoxygenated blood from the body and pumps it into the right ventricle. Right ventricle The heart's chamber that receives blood from the right atrium and pumps it into the pulmonary artery, sending it to the lungs for oxygenation. Sino-atrial node The natural pacemaker of the heart, located in the right atrium, responsible for initiating the electrical impulses that regulate the heart's rhythm. Systole The phase of the cardiac cycle when the heart muscle contracts and pumps blood into the arteries. Systolic pressure The higher number in a blood pressure reading, representing the pressure in arteries during heartbeats What are the three layers of both the heart and the blood vessels? 1. Tunica Intima - innermost 2. Tunica Media - middle 3. Tunica Externa (adventitia) - outer What names do we use to refer to the three layers of the heart instead of the tunica? 1. Endocardium - tunica intima - epithelia lining the heart 2. Myocardium - tunica media - heart muscle 3. Epicardium - tunica adventitia - inner layer of the pericardium (visceral) What are the layers of pericardium? 1. Fibrous pericardium - outermost 2. Serous pericardium - parietal layer 3. Serous pericardium - visceral layer, epicardium What is meant by endothelium? The endothelium is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels . What constitutes the endocardium? Endothelium and the subepithelial connective tissue Are endothelial cells the same as epithelial cells Endothelium cells are a type of epithelial cells that line the bodies vessels lymph and blood Etymology of myocardium Ancient greek - mys for muscle become myo and kardia for heart became cardium Characteristics of cardiac muscle • Striated • Involuntary • Central nuclei • Cells are often elongated and branched • Tightly connected by intercalated discs • Intercalated dics contains gap junctions, desmosomes, and adhering junctions What do the gap junctions, desmosomes, and adhering junctions of the myocardium create? A functional syncytium What is the difference between a true syncytium and a functional syncytium? A functional syncytium is a group of individual cells that remain physically separate but function as a single coordinated unit, typically connected through gap junctions for the synchronized transmission of electrical or metabolic signals, whereas, a true syncytium is a single multinucleated cell resulting from the fusion of individual cells, with continuous cytoplasm and multiple nuclei. Eg true syncytium Skeletal muscle cell Eg functional syncytium Cardiac muscle cells What makes up the epicardium? Mesothelium and connective tissue Mesothelium Mesothelium is a type of tissue composed of a layer of flat cells known as mesothelial cells, which line the body's serous cavities, including the pleural, pericardial, and peritoneal cavities. These cells secrete a lubricating fluid that reduces friction between the internal organs and the cavity walls, allowing them to move smoothly within the body. What forms the heart valves? • Formed by thin folds of endocardium • With dense fibroelastic connective tissue at the core • Endocarditis - infection of valves that can lead to faulty valves and the formation of clots that can cause strokes and heart attacks - linked to poor oral health What is the fibrous cardiac skeleton? Four fibrous rings of dense irregular connective tissue that make up the fibrous core of the heart -> insulates electrically atria from ventricles below and is the attachment site for myocardium and the heart valves What are purkinje fibres? Modified cardiac muscle cells specialised for conduction: • subendocardial • stain differently • they are larger than other cardiac muscle cells • pale cytoplasm • contain more glycogen • few myofibrils and these are mainly located at the cell periphery • no intercalated discs The epicardium What is the tunica media continuous with? The myocardium What is the tunica externa or the adventitia continuous with? The pericardium Tunica intima • Endothelium - simple squamous epithelium • Basement membrane • Connective tisue - subendothelial layer • Sometimes an internal elastic lamina Tunica media Mainly concentric layers of smooth muscle and elastin sometimes combined with an external elastic lamina Tunica externa/adventitia • Collagen rich connective tissue • Simple squamous epithelium • Larger vessels: lymphatics, nerves, and vasa vasorum - vessel of the vessels What is different about the tunica media in arteries and veins? In arteries, due to them being under higher pressure than veins, the tunica media is broader. What are elastic arteries? Major arteries than come off the heart very quickly, e.g. the aorta, the carotid, and the pulmonary What is the specialised histology of elastic arteries? • Tunica media - very broad with lots of elastic fibres and collagen • Elastic arranged in concentric layers • A few smooth muscle cells What is the specialised function of elastic arteries? • Elastin allows expansion • Elastic allows recoil and hels maintain arterial pressure during diastole What does a Van Gieson stain of an elastic artery show? The elastin in the elastic of the arteries stains black showing how they are present in lots and lots of concentric circle layers What are the distinguishable features of the elastic arteries? • Many elastic lamina • Fenestrations of elastic tissue • Broad tunica media What is ageings impact on elastic arteries? With ageing they lose their elastic tissue which leads to an increase in arterial resistance and an increase in arterial blood pressure Muscular arteries eg Femoral, coronary Muscular artery lumen size 1-10mm What is the specialised function of a muscular artery? Smooth muscle changes vessel diameter to control blood flow to organs What is the specialised histology of muscular arteries? Tunica media has more smooth muscle cells present than elastic arteries with up to 40 layers What is the distinguishable feature of muscular arteries? Internal elastic lamina and external elastic lamina present between tunica media What is the specialised function of arterioles? Control blood flow to capillaries by vasoconstriction and control blood resistance and pressure at the periphery What is the specialised histology of arterioles? Small artery with small lumen with 1-3 layers of smooth muscle in tunica media. There is an internal elastic lamina that is present only in large arterioles. What is the specialised function of capillaries? Connect arterioles to venules and exchange materials across thin barriers What is the specialised histology of capillaries? • Single thin layer of endothelial cells - only part of the tunica intima present no media or adventitia • supportive pericytes present What are the three major types of capillaries? 1. Continuous 2. Fenestrated 3. Sinusoid - discontinuous Features of continuous capillaries • No pores in endothelial cells • On complete basement membrane • Pinocytic vesicles Features of fenestrated capillaries • Fenestrae - pores 60-80 mm • Complete basement membrane What are the features of sinusoid capillaries? • Large diameter • Large fenestrations • Discontinuous basement membrane permeable to larger macromolecules • Permeable to larger molecules What is the specialised function of venules to medium veins? • Post-cap venules allow blood return • WBC usually leave the blood stream here • Less muscular and elastic content so the lumen is collapsable • Valves made from fibroelastic tissue prevent backflow What blood vessel are lymph vessels similar to? Veins How are lymph vessels different to veins? Similar to veins but they have thinner and less distinct layers What are lymph capillaries? Thin closed ended vessels - fenestrated endothelium What is ateriosclerosis? Arterial stiffening characterised by: • tunica intima increases in size (intimal proliferation) • stiffens with age • can occur in normal ageing • may be a part of atherosclerotic plaque formation What is Marfan's syndrome Genetic disease characterised by: • mutations in Fibrillin gene • causes a decrease in functional elastic fibres and therefore a decrease of recoil • increased luminal diameter and wall weakening in Marfan's can lead to aneurysm, aortic dissection/rupture risk What can replace elastic fibres in blood vessels in diseases? Diseases such as ateriosclerosis, atherosclerosis, Marfan's syndrome and EhlersDanlos syndrome can lead to elastic fibres being replaced with collagen. What are diads in cardiac muscle and how do they compare with triads in skeletal muscle? Diads in cardiac muscle and triads in skeletal muscle are structural components involved in calcium ion regulation for muscle contraction. Diads in cardiac muscle consist of a T-tubule and a single terminal cisterna of the sarcoplasmic reticulum, enabling coordinated contractions in the heart. In contrast, triads in skeletal muscle comprise a T-tubule between two terminal cisternae of the SR, facilitating powerful and voluntary muscle contractions. While both serve similar functions, these structures have structural differences that suit the specific needs of cardiac and skeletal muscle. What surrounds necrotic cardocytes after a MI? Three days later after an MI neutrophils surround it Where is the internal elastic lamina located in muscular arteries? Internal Elastic Lamina: The internal elastic lamina is found in the tunica intima, the innermost layer of the arterial wall. It separates the tunica intima from the tunica media, which is the middle layer. This layer is primarily composed of elastic fibers and acts as a boundary between the intima and media. Where is the external elastic lamina located in muscular arteries? The external elastic lamina is located between the tunica media (middle layer) and the tunica adventitia (outer layer) of the arterial wall. It is also composed of elastic fibers and provides structural support to the artery. The external elastic lamina is not as prominent as the internal elastic lamina but is present in many muscular arteries Why does the mitral valve close? The mitral valve closes during ventricular contraction (systole) to prevent the backflow of blood from the left ventricle to the left atrium. This closure is driven by the pressure difference in the cardiovascular system, where the left ventricular pressure becomes higher than the left atrial pressure. It ensures oneway blood flow, from an area of higher pressure (left ventricle) to an area of lower pressure (aorta and the rest of the systemic circulation). Why does the aortic valve open? The aortic valve opens when the left ventricular pressure becomes higher than the pressure in the aorta. This pressure difference allows the aortic valve to open, enabling the ejection of oxygenated blood from the left ventricle into the aorta, from where it is distributed to the body's systemic circulation. Why does the aortic valve close and how does its closing differ from the mitral valve? The aortic valve closes when the left ventricular pressure falls below the pressure in the aorta during diastole. This closure prevents the backflow of blood from the aorta back into the left ventricle. Unlike the mitral valve, which closes to prevent backflow into the left atrium during ventricular systole when left ventricular pressure is higher, the aortic valve closes during diastole to prevent regurgitation into the left ventricle when the aortic pressure exceeds the left ventricular pressure. This distinction in timing and pressure gradients ensures the one-way flow of blood in the circulatory system. Why does the mitral valve open? The mitral valve opens during ventricular diastole when the left atrial pressure exceeds the left ventricular pressure. This pressure difference allows the mitral valve to open, facilitating the flow of oxygenated blood from the left atrium into the left ventricle, which is a crucial step in the filling of the left ventricle before the next systolic contraction. What is end diastolic volume (EDV)? End-diastolic volume (EDV) is the volume of blood present in the left ventricle at the end of the diastolic phase of the cardiac cycle, just before the ventricle contracts (systole). It represents the maximum amount of blood the left ventricle can hold at the end of its relaxation and filling phase, which occurs during diastole. EDV is an important parameter in assessing cardiac function and is often used in calculations to determine measures like stroke volume and ejection fraction. What is end systolic volume (ESV)? End-systolic volume (ESV) is the volume of blood remaining in the left ventricle at the end of the systolic phase of the cardiac cycle, just after the ventricle has contracted and ejected blood into the aorta. It represents the minimum amount of blood left in the ventricle at the end of systole. ESV is an essential parameter used to calculate stroke volume, which, when combined with the end-diastolic volume (EDV), helps determine the ejection fraction, a measure of the heart's pumping efficiency. Describe the relationship between arterial blood pressure (ABP), stroke volume (SV), heart rate (HR), and total peripheral resistance (TPR). ABP = SV x HR x TPR What is atrial systole? The first stage of the two sub-phases of the end of diastole. This sub-phase occurs towards the end of diastole. It is the period during which the atria contract to push the remaining blood into the ventricles. Atrial systole contributes to the filling of the ventricles and ensures that they are maximally filled before ventricular contraction (systole). What is isovolumetric contraction? The second phase of the end of diastole. Immediately following atrial systole, there is a brief period known as isovolumetric contraction. During this sub-phase, both the atrioventricular (AV) valves (e.g., the mitral and tricuspid valves) and the semilunar valves (e.g., the aortic and pulmonic valves) are closed. The ventricles begin to contract, generating pressure, but no blood is ejected yet. This phase is called "isovolumetric" because the volume of blood in the ventricles remains constant. What is the conduction speed of the atrial muscle? 0.5 m/s What is the conduction speed of the AV node? 0.05 m/sec What is the conduction velocity of the Bundle of His? 2 m/s What is the conduction velocity of the left and right bundle branches? Both are the same - 2 m/sec What is the conduction velocity of the ventricular muscle? 0.5 m/s What is the conduction velocity of the Purkinje Fibres? 4 m/sec Describe the proceedings of the cardiac cycle 1. 2. 3. 4. Electrical activity Mechanical activity Pressure changes Volume changes What is the cardiac cycle? The movement of blood through the heart due to pressure changes generated by mechanical activity What is echocardiography used for? To assess the volume and function of the heart What happens to the length of the cardiac cycle when heart rate increases? It will decrease and vice versa Which, out of systole and diastole, shortens the most when heart rate increases? Diastole What is the majority of filling? It is passive - doesn't require atrial contraction to push blood into the ventricles What is systole? When the ventricle is contracting resulting in pressure being generated so that ejection into either the pulmonary artery or aorta can occur What preportion of the cardiac cycle is systole? 1/3rd What will contraction of the muscles of a chamber of the heart do? It will increase the pressure within that chamber What happens to the pressure in a chamber of the heart when that chamber's muscle relaxes? It drops rapidly What is the amount of pressure generated in a chamber of the heart dependent on? The thickness of the muscle around it, therefore left ventricular pressure is greater than right ventricular pressure cuz LV muscle is thicker When will heart valves open? When there is a pressure/energy gradient across them, e.g. the right atrioventricular valve will open when the pressure in the right atrium exceeds that of the pressure in the right ventricle. What does blood flow down? A pressure/energy gradient from an area of higher pressure to lower pressure What happens to the pressure changes in adjacent chambers of the heart when the valves are open? They change together When valves are closed what can happen to the pressures in adjacent chambers of the heart? They are independent of each other and so can be different What is the average resting heart rate? 70 bpm What is the average length of a cardiac cycle at resting heart rate? 850ms What is the length of diastole at average resting heart rate? 600 ms What is the length of systole at average resting heart rate? 250 ms What offsets the reduction in passive filling time seen in diastole when heart rate increases? Stimulation of the atrial wall muscle to contract with greater force. What is cardiac output? The volume of blood pumped by the heart per minute Cardiac output equation? HR (heart rate/bpm) x stroke volume Stroke volume Volume of blood ejected from the ventricle with each contraction Stroke volume calculation SV = end diastolic volume - end systolic volume EDV approx value at rest 120ml ESV approx value at rest 50ml Stroke volume at rest 70ml Cardiac output approx at rest 5l/min EDV End diastolic volume - volume in the ventricle at the end of diastole ESV End systolic volume - volume in the ventricle at the end of systole How can you increase stroke volume? By either increasing EDV or decreasing ESV Why is the fact that the heart doesn't eject all the blood it contains with each beat? Because it provides a reserve of blood which allows the stroke volume to be adjusted on a beat by beat basis depending on the needs of the body What are textbook values of cardiac function based on? 21 yr old male Describe the stages of the cardiac conduction system 1. 2. 3. 4. 5. sinoatrial node Electrical spread through atria Atrioventricular node delay Conduction along his bundles and purkinje fibres Electrical spread from ventricular endocardium out to the epicardium Which of the areas of the heart have spontaneous AP generation? SAN, AVN, and Purkinje fibres SAN AP/min 80-100 What is the shape of the SAN AP What ions are responsible for the 1st phase of the SAN pacemaker potential? 1st phase What ions are responsible for the second phase of the SAN AP? 3nd phase of SAN AP Describe the innervation of the SAN If the spontaneous AP generation rate is 80-100 AP/min, why is the resting heart rate 60-70 bpm? Because vagal tone predominates and slows down our heart rate What is parasympathetic vagal stimulation of the SAN? What happens during sympathetic stimulation of the SAN? Spontaneous AVN AP/min? 40-60 What does the delay caused by the atrioventricular node lead to? ensures atrial depolarisation, contraction & ejection before ventricles depolarise Describe the shape of the AV node's action potential? What is the hierachy of cardiac pacemakers? Purkinje fibres spontaneous AP generation - AP/ min? 20-40 Phases of ventricular myocyte action potentials: Which ions are responsible for each stage of a ventricular myocytes action potential? Refractory periods Time from initial depolarisation of the first AP to the point at which a second AP can be stimulated What determines the refractory period? The number of available and recovered (re-primed) voltage gated sodium channels What is sodium channel recovery dependent on? Time and voltage Does a more negative or more positive membrane potential cause the sodium channels to recover faster? More negative membrane potentials What causes the cardiac myocytes to be electrically coupled? The presence of gap junctions What do gap junctions between the cardiac myocytes do? They electrical couple cardiac myocytes by allowing the passage of positively charged ions between them if there is a charge gradient Describe how the electrical stimulation is conducted across the cardiac myocytes: 1. Before the electrical stimulation reaches the cells (cell 1 and 2) both cells are at a negative resting membrane potential 2. Therefore, there is no charge gradient 3. So, no movement of ions (sodium ions and chloride ions) between the cells 4. However, when the electrical stimulation reaches cell 1, sodium influx into it during depolarisation causes the membrane potential to become more positive (+20mv) 5. This creates a large charge gradient between cell 1 and 2 6. So, there is movement of positive ions from cell 1 to cell 2 through gap junctions 7. This causes cell 2 to become more positive 8. When it reaches threshold (around -60mV) enough voltage gated sodium channels open that an action potential is triggered in cell 2 What determines conduction velocity? • The charge gradient between cells • Set by the magnitude of depolarising current, i.e. the action potential amplitude • This varies in disease leading to arrhythmia How can conduction velocity be modified? Gap junction expression and function Where is the sino-atrial node located? at the junction of the crista terminalis in the upper wall of the right atrium and the opening of the superior vena cava . Where is the AV node located? Posteroinferior interatrial septum wall, within triangle of atrioventricular node (Koch’s triangle) What is the cardiac cycle? The movement of blood through the heart and out the great vessels due to pressure changes generated by the muscular mechanical activity of heart. How can the relationship between the arterial pressure, stroke volume, heart rate, and resistance be summarised? ABP = SV x HR x TPR What is the resistance that the left side of the heart has to overcome? Systemic resistance - total peripheral resistance What is the resistance the right side of the heart has to overcome? Pulmonary vascular resistance How can the pressure and volume changes in the aorta and left ventricles be measured clinically? Introducing catheters into these structures What preportion of the cardiac cycle is diastole? 2/3rds What are the 5 principles of the cardiac cycle? 1. Pressure will increase in a chamber when muscle around it contracts 2. Valves will open when there is a pressure/energy gradient across them 3. Blood will flow down a pressure/enery gradient from an area of higher pressure/ energy to an area of lower pressure/energy 4. When valves are open, pressures in neighbouring chambers change together 5. When valves are closed, pressures in neighbouring chambers can be different Why is left ventricular pressure greater than right ventricular pressure? Because LV muscle wall is thicker so it can generate a higher pressure to overcome the larger total peripheral resistance Energy of blood is: Pressure x momentum End diastolic volume Volume left in heart at the end of diastole Cardiac cycle phases 1. Ventricular diastole 2. Ventricular systole Ventricular diastole 1. Isovolumetric relaxation 2. Rapid passive filling 3. Atrial systole Ventricular systole 1. Isovolumetric contraction 2. Ejection What is occuring in ventricular diastole? Ventricles filling with blood Describe the features of a pressure-volume loop (for the left ventricle) What is a Wiggers diagram? A Wiggers diagram is a graphical representation of a cardiac cycle that illustrates the key events in the cardiovascular system. It typically shows points for atrial and ventricular pressure, as well as lines representing electrical activity (ECG), heart sounds, and major phases like systole and diastole, helping visualize the sequence of events during one heartbeat. What happens during atrial systole? • Atrial Systole : • Atrial systole is the initial phase of the cardiac cycle. • During this stage, the atria contract, pushing blood into the relaxed ventricles. • Atrioventricular (AV) valves (tricuspid and bicuspid) open, allowing blood to flow from the atria into the ventricles. • The semilunar valves (pulmonary and aortic) are closed. What happens during isovolumetric ventricular contraction? • Isovolumetric Ventricular Contraction : • This phase follows atrial systole. • The ventricles start to contract, increasing pressure. • All heart valves are closed, so no blood is ejected yet. • Pressure builds within the ventricles. What happens during ventricular ejection? • Ventricular Ejection : • As ventricular pressure exceeds the pressure in the pulmonary artery and aorta, the semilunar valves open. • Blood is ejected from the ventricles into the pulmonary artery and aorta. • This is the phase responsible for pumping oxygenated blood to the body (left ventricle) and deoxygenated blood to the lungs (right ventricle). What happens during isovolumetric ventricular relaxation? • Isovolumetric Ventricular Relaxation : • After ejection, the ventricles begin to relax. • All heart valves close briefly to prevent blood backflow into the ventricles. • This phase marks the end of systole. What happens during atrial diastole? • Atrial Diastole : • During this stage, the atria are relaxed and fill with blood. • Pressure in the atria exceeds that in the ventricles, causing the AV valves to open. • Blood flows from the atria into the ventricles, preparing for the next cycle. What is the main function of isovolumetric relaxation? To reduce ventricular pressure below atrial pressure so the AV valve can open and ventricular filling can start What is the small peak in atrial pressure that occurs during ventricular contraction due to? AV valve pressing back into atria What does the wiggers diagram for the left ventricle look like? Normal pressure in the aorta 120/80 Normal pressure in the RA 4 Normal pressure in the right heart 25/4 Normal pressure in the PA 25/10 Normal pressure in the LA 8 Normal pressure in the LV 120/8 Flow = pressure/resistance Flow = cardiac output P-wave • The P-wave represents atrial depolarization, the electrical activation of the atria. • It signifies the initiation of the heartbeat and the contraction of the atria. QRS Complex • The QRS complex represents ventricular depolarization, the electrical activation of the ventricles. • It is a crucial part of the ECG because it signifies the contraction of the ventricles, which is responsible for pumping blood to the lungs and the rest of the body. • Duration (HR of 60bpm) = 0.08 s (80 ms) • The amplitude of the QRS complex indicates the amount of ventricular muscle. ◦ A QRS complex with large amplitudes (>3.5mV) may be explained by ventricular hypertrophy or enlargement (or both). T-wave • The T-wave represents ventricular repolarization, the recovery of the ventricles to their resting state after depolarization. • It marks the period when the ventricles are preparing for the next heartbeat. PR interval • The PR interval is the time from the beginning of the P-wave to the start of the QRS complex. • It indicates the time it takes for the electrical signal to travel from the atria to the ventricles, including the delay at the atrioventricular (AV) node. ST segment • The ST segment is the flat, isoelectric line that follows the QRS complex and precedes the T-wave. • It represents the interval between ventricular depolarization and repolarization. • Changes in the ST segment can indicate myocardial ischemia or injury. QT interval • The QT interval represents the time from the start of the QRS complex to the end of the T-wave. • It reflects the total time for ventricular depolarization and repolarization. • Prolongation of the QT interval can indicate an increased risk of arrhythmias • Duration (HR 60bpm) = 0.40 s (400 ms) U-wave • The U-wave is a small, sometimes observable wave following the T-wave. • Its exact origin and significance are not entirely understood, but it may be related to repolarization of Purkinje fibers or electrolyte imbalances. How many heart sounds are there? 4 What are the 4 heart sounds? • S1 • S2 • S3 • S4 What is the 1st heart sound? • S1 (Lub) : • S1 is the first heart sound and is often described as "lub." • It is produced by the closure of the atrioventricular (AV) valves (the tricuspid and bicuspid/mitral valves). • S1 marks the beginning of ventricular systole, signifying the onset of ventricular contraction. • S1 is associated with the start of the ejection of blood from the ventricles into the pulmonary artery and aorta. What is the second heart sound? • S2 (Dub) : • S2 is the second heart sound and is often described as "dub." • It is produced by the closure of the semilunar valves (the aortic and pulmonary valves). • S2 marks the end of ventricular systole and the beginning of diastole, signifying the cessation of ventricular contraction and the start of ventricular relaxation. • S2 is associated with the closure of the aortic and pulmonary valves as blood is forced out of the ventricles into the systemic and pulmonary circulations, respectively. What is the third heart sound? • S3 (Third Heart Sound) : • S3 is an additional heart sound that can sometimes be heard, but it is not always present. • It occurs early in diastole and is associated with rapid ventricular filling when the ventricles relax. • S3 can be a sign of heart failure or volume overload in the ventricles. What is the 4th heart sound? • S4 (Fourth Heart Sound) : • S4 is another extra heart sound that is not always present. • It occurs late in diastole and is associated with atrial contraction just before ventricular systole. • S4 can be indicative of conditions like hypertensive heart disease, aortic stenosis, and ventricular noncompliance. What is the left atrioventricular valve? • Otherwise known as the mitral or bicuspid valve • two cusps - anterior (aortic) and posterior (mural) • associated with inferior (posterior) and superior (anterolateral) papillary muscles. • prevents blood flow from left ventricle into left atrium What is the right atrioventricular valve? • tricuspid valve • three cusps - anterior/superior, septal, and posterior/inferior • associated with four papillary muscles - anterior, septal (medial), inferior (posterior), and moderator band (septomarginal trabecular) • prevents blood from flowing from the right ventricle into the right atrium What is the pulmonary valve? • Three semilunar cusps - anterior (non-adjacent), right (right adjacent), and left (left adjacent) • No associated papillary muscles • Prevents backflow of blood from pulmonary circulation into the right ventricle What is the aortic valve? • Three semilunar cusps - right coronary (right semilunar), left coronary (left semilunar), and a non-coronary cusp (posterior semilunar). • No associated papillary muscles • Prevents backflow of blood from systemic circulation into the left ventricle What is the effect of parasympathetic innervation on the heart? Vagal stimulation: • Slowing of heart rate • Reduced force of contraction • Decreased atrioventricular node conduction • Vasodilation What does the balance between the sympathetic and parasympathetic inputs to the heart do? Maintain heart rate and cardiac function within a range that meets the body's physiological needs under different circumstances What is the origin of the parasympathetic innervation to the heart? Cranial sacral - mainly vagus but some S2-S4 What is the effect of sympathetic innervation to the heart? • Increased heart rate • Increased force of contraction • Dilation of coronary arteries • Enhanced conduction • Activation of the Beta-1 Adrenergic receptors What does the activation of Beta-1 Adrenergic Receptors by the sympathetic nervous system do on the heart? This is the pathway that mediates the effects of sympathetic innervation on the heart. These receptors are found on the surface of cardiac cells and are responsive to noradrenaline. Activation increases heart rate, contractility, and conduction speed. Spinal levels of the sympathetic innervation to the heart? T1-T5 What are the two different types of heart valves? Atrioventricular valves and semilunar valves They differ in structure and location Location: AV valves - between atria and ventricles Semilunar valves - between ventricles and arteries (aorta and pulmonary) Structure: Semilunar -Semilunar valves have three pocket-like, semilunar-shaped cusps (hence the name "semilunar"). These cusps are thin and do not contain chordae tendineae or papillary muscles, unlike the AV valves. AV - AV valves have two or three cusps, but they are thicker and more muscular than the cusps of semilunar valves. They also contain chordae tendineae and papillary muscles, which help prevent valve prolapse during ventricular contraction. Which side of the heart is deoxygenated blood brought too? Right Which side of the heart is oxygenated blood brought too? Left At what level does the common carotid artery bifurcate? C4/5 - around the level of the superior border of the thyroid cartilage When does the subclavian artery become the axillary artery? When it passes the lateral border of the first rib When does the axillary artery become the brachial artery? At the inferior border of the terres major muscle? At what point does the brachial artery bifurcate into the ulnar and radial arteries? 1cm distal to the elbow What is meant by collatoral blood supply? Collateral blood supply refers to the alternative or backup pathways through which blood can flow to a particular tissue or organ when the primary blood vessels supplying that area become partially or completely blocked. How many palmer arches are there? 2 What are the two palmer arches? 1. Superficial 2. Deep What is the superficial palmer arch? This arch is located closer to the surface of the palm and is formed primarily by the ulnar artery. The ulnar artery is one of the two main arteries in the forearm, and it enters the hand from the medial (inner) side. The superficial palmar arch provides blood supply to the superficial structures of the palm and fingers. What is the deep palmer arch? The deep palmar arch is located deeper within the palm and is primarily formed by the radial artery, the other major artery in the forearm. The radial artery enters the hand from the lateral (outer) side. The deep palmar arch provides blood supply to deeper structures of the palm and fingers. How are the two palmer arches connected and why is this important? These two palmar arches are interconnected through a network of smaller arteries and anastomoses (connections) between them. This interconnected network allows for a continuous and balanced blood supply to the hand, ensuring that the hand's muscles, bones, tendons, and other structures receive adequate oxygen and nutrients. What is the dorsal venous arch? The dorsal venous arch, also known as the dorsal venous network or dorsal venous plexus, is a network of veins located on the dorsal (back) surface of the hand and the top of the foot. It plays a crucial role in venous return, which is the process of carrying deoxygenated blood back to the heart after it has circulated through the body's tissues. In the hand, what veins feed into the dorsal venous arch? In the hand, the dorsal venous arch receives blood from the digital veins of the fingers and the dorsal metacarpal veins. What does the dorsal venous arch drain into (hand)? The cephalic vein and basilic vein What does the dorsal venous arch of the foot drain into? The great saphenous vein Origin of the cephalic vein In the hand, the dorsal venous arch receives blood from the digital veins of the fingers and the dorsal metacarpal veins. Course of the cephalic vein The cephalic vein runs along the lateral (outer) side of the arm, following a course that is somewhat parallel to the upper arm bone, the humerus. It ascends along the forearm and into the upper arm. The vein is often visible through the skin, making it a common choice for venipuncture (inserting a needle into a vein for various medical procedures). Termination of the cephalic vein The cephalic vein typically terminates by draining into the axillary vein. This convergence of the cephalic vein into the axillary vein usually occurs in the region of the shoulder, where the axillary vein is formed as the continuation of the brachial vein. The axillary vein eventually merges with other veins, such as the subclavian vein, leading to the superior vena cava. The superior vena cava carries deoxygenated blood back to the heart for circulation to the lungs and the rest of the body. Origin of the basillic vein Arises from the dorsal venous network of the hand and forearm, and it is often a companion vein to the cephalic vein. Course of the median vein Ascends along the medial (inner) aspect of the arm. Termination of the basilic vein Typically drains into the brachial vein. Origin of the brachial vein Forms from the union of the basilic vein and the profunda brachii vein. Course of the brachial vein Courses along the upper arm, paralleling the brachial artery. Termination of the brachial vein Drains into the axillary vein. Origin of the axillary vein Formed by the union of the brachial vein and the basilic vein. Course of the axillary vein Passes through the axilla (armpit) region. Termination of the axillary vein The transition from the axillary vein to the subclavian vein typically occurs near the outer border of the first rib, which is located at the junction of the shoulder and the upper chest. This region is known as the axillary-subclavian junction. Course of the subclavian vein Descends from the shoulder region toward the upper chest. Termination of the subclavian vein Drains into the brachiocephalic vein. What is the median cubital vein? A bridge or connection between two other important veins in the arm, the cephalic vein (which travels along the lateral or outer part of the forearm and arm) and the basilic vein (which runs along the medial or inner aspect of the forearm and arm). Why is the median cubital vein often chosen for venipuncture? The median cubital vein is often chosen for venipuncture because it is usually prominent, easy to locate, and less likely to roll or move during the procedure. It provides good access to the venous system and is commonly used for drawing blood samples or administering medications through an IV line. What is the significance of the cubital fossa? • Common site for venipuncture (blood draw) and IV access. • Used for blood pressure measurement. • Contains brachial artery, median nerve, and radial nerve. • Key anatomical landmark for the elbow's structures. Cubital fossa location Triangular depression at the front (anterior aspect) of the elbow. What are the unpaired branches of the abdominal aorta? • Coeliac trunk • Superior mesenteric artery • Inferior mesenteric artery What vertebral level is the coeliac trunk given off from the abdominal aorta? T12 What vertebral level is the superior mesenteric artery given off? L1 What vertebral level is the inferior mesenteric artery given off the abdominal aorta? L3 What is the foregut supplied by arterially? Coeliac trunk What is the midgut's arterial supply? Superior mesenteric artery What is the arterial supply to the hingut? Inferior mesenteric artery What are the paired arteries that come off the abdominal aorta? Renal arteries and the gonadal arteries Midgut boundaries The midgut begins at the distal part of the duodenum (the point where the bile duct and main pancreatic duct enter) and extends to the proximal two-thirds of the transverse colon. Foregut boundaries The foregut begins at the oral cavity (mouth) and extends down to the proximal part of the duodenum. Hindgut boundaries 1. • the hindgut encompasses the distal one-third of the transverse colon, the descending colon, the sigmoid colon, and the upper part of the rectum. What is the venous drainage from the gi tract? Portal venous system to the liver What are the boundaries of the femoral triangle? Base - inguinal ligament Lateral border - sartorius Medial border - adductor longus What are the contents of the femoral artery? • femoral nerve • femoral artery • femoral vein • inguinal lymph nodes At what anatomical boundary does the external iliac artery become the femoral artery? Inguinal ligament What is the importance of the femoral artery? It is the principle blood suppy to the lower limb At what anatomical boundary does the femoral artery become the popliteal artery? Adductor hiatus What are the boundaries of the popliteal fossa? • Superior Border: Femur's lower end (superiorly) • Inferior Border: Soleus muscle (inferiorly) • Medial Border: Semimembranosus and semitendinosus tendons (medially) • Lateral Border: Biceps femoris muscle (laterally) What are the contents of the popliteal fossa? • Popliteal Artery • Popliteal Vein • Tibial Nerve • Common Fibular (Peroneal) Nerve • Small Saphenous Vein What is the genicular anastomosis? A network of arteries that provides collateral circulation around the knee joint. What are the key arteries of the genicular anastomosis? Branches of the femoral, popliteal, and anterior and posterior tibial arteries. What is the purpose of the genicular anastomosis? Crucial for maintaining blood supply to the knee, especially during occlusion or injury. What is the clinical significance of the genicular anastomosis? Crucial for maintaining blood supply to the knee, especially during occlusion or injury. What are the 10 important arteries of the pelvis? 1. Common iliac 2. Internal iliac/hypogastric 3. 4. 5. 6. 7. 8. 9. 10. External iliac Uterine (female) Superior vesical artery Inferior vesical artery (male) or vaginal artery (female) Middle rectal artery Obturator Internal pudendal Common hepatic or gastroduodenal Common Iliac Arteries • • Location: Bifurcation of the abdominal aorta • Function: Supply blood to the pelvis and lower limbs Internal Iliac Arteries (Hypogastric Arteries): • • Location: Branch from common iliac arteries and enter the pelvis • Function: Primary supply to pelvic organs, rectum, bladder, and pelvic wall muscles External Iliac Arteries: • • Location: Continue from common iliac arteries toward the lower limbs • Function: Supply blood to the lower extremities Uterine Artery (in females): • • Location: Branch from internal iliac artery • Function: Supplies blood to the uterus Superior Vesical Artery: • Location: Arises from the internal iliac artery • Function: Supplies the superior part of the bladder Inferior Vesical Artery (in males) or Vaginal Artery (in females): • Location: Arises from the internal iliac artery • Function: Supplies the lower part of the bladder, prostate (in males), or vaginal structures (in females) Middle Rectal Artery: • Location: Branch from the internal iliac artery • Function: Supplies blood to the rectum Obturator Artery: • Location: Travels through the obturator foramen • Function: Supplies the muscles and structures in the pelvic wall Internal Pudendal Artery: • Location: Branch from the internal iliac artery • Function: Supplies the perineum, external genitalia, and rectum Femoral arteries The femoral arteries are the primary arteries of the thigh. They give rise to various branches, including the deep femoral artery (profunda femoris) and the descending genicular arteries, which supply the muscles of the thigh and the knee joint. Popliteal Artery: The popliteal artery is located behind the knee joint in the popliteal fossa. It is a continuation of the femoral artery and provides blood to the muscles, bones, and other structures in the leg. Anterior Tibial Artery: This artery is a branch of the popliteal artery and runs down the front of the leg. It becomes the dorsalis pedis artery on the dorsum of the foot. Posterior Tibial Artery: This artery is also a branch of the popliteal artery and travels down the back of the leg. It supplies blood to the calf muscles and forms the medial and lateral plantar arteries in the foot. Peroneal Artery (Fibular Artery): The peroneal artery is another branch of the popliteal artery. It runs along the lateral aspect of the leg and provides blood supply to the lateral compartment of the leg. Dorsalis Pedis Artery: The dorsalis pedis artery is a continuation of the anterior tibial artery and runs along the dorsum (top) of the foot. It is a key artery supplying the foot and toes. Medial and Lateral Plantar Arteries: These arteries originate from the posterior tibial artery and provide blood supply to the sole of the foot. Arcuate Artery: The arcuate artery is a network of smaller arteries that forms between the dorsal and plantar arteries of the foot, contributing to the overall blood supply of the entire foot. Perforating Arteries: These arteries link the dorsal and plantar arteries, creating a collateral network that ensures efficient blood distribution throughout the foot and ankle. Calcaneal Artery: This artery arises from the posterior tibial artery and supplies blood to the calcaneus (heel bone). Tarsal Arteries: Multiple smaller arteries branch from the major arteries mentioned above to provide blood supply to the tarsal bones in the ankle region. What important nervous system structures surround the axillary artery along its course through the axilla? • Surrounding Nerves: Brachial Plexus, Median Nerve, Ulnar Nerve, Radial Nerve • Role: Nerves control and provide sensation to the upper limb. • Significance: Close relationship with axillary artery is vital for arm and hand function. As the popliteal vein passes superiorly through the lower limb, it reaches the adductor hiatus. From thereon, what is it referred to as? The femoral vein What superficial vein drains into the popliteal vein at the popliteal fossa? Small saphenous vein Where does the small saphenous vein originate? the lateral aspect of the foot and runs up the posterior (back) of the leg. Through which space will the posterior tibial vein travel to enter the foot? The posterior tibial vein travels through the tarsal tunnel to enter the foot. What is the tarsal tunnel? The tarsal tunnel is a fibro-osseous space on the medial (inner) side of the ankle, and it contains various structures, including the posterior tibial artery, posterior tibial vein, tibial nerve, and tendons of several muscles. The posterior tibial vein, along with the posterior tibial artery, passes through this tunnel to supply blood to the posterior and plantar regions of the foot. What neurovascular structures accompany the posterior tibial vein through the tarsal tunnel? Posterior tibial artery and the tibial nerve Give three specific locations where superficial veins will join deep veins, naming the vessels involved. 1. Axilla: Cephalic Vein and Basilic Vein join with Axillary Vein. 2. Groin: Great Saphenous Vein and Small Saphenous Vein join with Femoral Vein. 3. Popliteal Fossa: Anterior Tibial Vein and Posterior Tibial Vein join with Popliteal Vein. Female vs male pelvic venous drainage • In females, venous drainage includes ovarian veins, uterine veins, and a vaginal venous plexus, specific to reproductive structures. • Ovarian veins drain the ovaries, while the uterine veins drain the uterus. • Differences in venous drainage patterns due to the presence of femalespecific reproductive organs. • In males, there are no equivalent structures to the ovarian veins, and the venous drainage patterns are distinct. • Recognizing these distinctions is vital for medical diagnosis and treatment, especially in the context of gynecological and urological conditions. What common procedures or treatments involve accessing the superficial veins of the upper limb? • Venipuncture for blood tests. • IV cannulation for medication and fluid administration. • Phlebotomy for therapeutic blood removal. • Blood transfusion through superficial veins. • Chemotherapy via IV lines. • IV medications in emergency or critical care. • Intravenous contrast for diagnostic imaging. • Non-invasive blood pressure measurement. • Arterial blood gas sampling for blood analysis. What is the clinical significance of the great saphenous vein? • Common site for varicose vein assessment and treatment. • Evaluated for venous reflux and insufficiency. • Used as a graft in coronary artery bypass surgery. • Relevant in managing lower limb edema and venous ulcers. • Considered in cases of deep vein thrombosis (DVT). • Important in aesthetic sclerotherapy for varicose veins. • Key vessel in vascular medicine and surgery. What are varicose veins and how can they be treated? • Varicose veins are swollen, twisted, and often painful veins, typically in the legs. • Causes include damaged vein walls, aging, hormonal changes, prolonged sitting/standing, and obesity. • Symptoms may include visible veins, discomfort, swelling, and skin changes. • Treatments range from lifestyle changes and compression stockings to minimally invasive procedures (sclerotherapy, EVLT, radiofrequency ablation) and, in severe cases, surgical removal. What is collateral circulation? Give 2 examples, naming the relevant vessels, discussing their functional relevance. • Definition: Alternative blood vessels that provide backup routes when primary vessels are blocked. • Example 1 (Coronary): In the heart, collateral vessels from different coronary arteries help maintain blood supply and reduce heart tissue damage during artery blockages. • Example 2 (Cerebral): Collateral circulation in the brain, such as the circle of Willis, redistributes blood flow, protecting against brain damage during arterial blockages. Abdominal aortic aneurysm • Definition: Abnormal dilation of the abdominal aorta. • Location: Typically below the renal arteries. • Risk Factors: Age, smoking, atherosclerosis, hypertension, and family history. • Symptoms: Often asymptomatic, but can cause abdominal or back pain. • Complications: Rupture is a major concern, leading to life-threatening bleeding. • Diagnosis: Imaging tests (ultrasound, CT, MRI) assess size and condition. • Management: Treatment varies based on size and symptoms, including monitoring, surgery, or stent graft placement. • Prevention: Lifestyle changes can help reduce the risk and prevent AAA development. How can the force of contraction of cardiac tissue be increased? 1. Increasing the calcium sensitivity of the contractile apparatus 2. Increasing the concentration of calcium in the cell What factors affect venous return to the right ventricle? 1. 2. 3. 4. 5. Blood volume Skeletal muscle pump Respiratory pump Venous tone Gravity Where is the blood volume distributed? 1. 2. 3. 4. 5. Systemic veins and venules - 60-70% Pulmonary circulation - 10-12% Systemic arteries - 10-12% Heart - 8-11% Systemic capillaries - 4-5% What does an increased blood volume (as seen in renal failure) lead to? Increased venous return, increased right ventricular filling, and increased stroke volume What does decreased blood volume (as seen in dehydration) lead to? Decreased venous return, decreased end diastolic volume, and decreased stroke volume Why is the fact that the heart doesn't eject all the blood important? Because it provides a reserve of blood Define excitation-contraction coupling The process by which an electrical signal (action potential) triggers a mechanical response (contraction) in cardiac muscle cells. Calcium-induced calcium release In order for the myosin heads to interact with the myosin binding site on actin, the regulatory troponin complex must undergo a conformational change to move out of the way. This occurs through calcium-induced calcium release: • calcium enters the cardiac muscle cells during the action potential via L type calcium channel • this stimulates the release of calcium from the sarcoplasmic reticulum via ryanodine receptor which is the calcium release channel on the sarcoplasmic reticulum • it is the calcium that is released from the sarcoplasmic reticulum that binds to troponin C and moves the regulatory complex out of the way allowing actin and myosin to form cross bridges and for contraction to occur • as such, it is calcium that regulates the number of actin-myosin crossbridges formed and therefore the force of ventricular contraction How can the force of contraction be increased? Increasing force of contraction means increasing the number of actin-myosin cross-bridges formed, and this can be accomplished by: 1. increasing the calcium sensitivity of the contractile apparatus so you get more cross-bridges formed for a given contraction of intracellular calcium 2. increasing the concentration of intracellular calcium in the cell How does cardiac muscle relaxation occur? When calcium is pumped back into the sarcoplasmic reticulum via SERCA which is regulated by phospholamban What is SERCA? Sacro endoplasmic reticulum calcium ATPase Define Starling's Law of the Heart A physiological principle that states that the force of cardiac contraction (stroke volume) increases as the volume of blood filling the heart (end-diastolic volume) increases, up to a point. Explain Starling's Law of the Heart • Starling's Law of the Heart, also known as the Frank-Starling mechanism, describes the relationship between the volume of blood entering the heart's chambers (ventricles) and the force of contraction during systole. • It suggests that as the volume of blood returning to the heart (preload) increases, the cardiac muscle fibers are stretched, leading to a more forceful contraction. • This increased force of contraction results in a greater stroke volume, which is the amount of blood ejected from the heart with each beat. • Starling's Law helps the heart adapt to varying conditions and maintain an adequate cardiac output to meet the body's demands. • However, there is an upper limit to this relationship. Beyond a certain point, excessive stretching of the cardiac muscle fibers can impair contraction, reducing stroke volume and cardiac efficiency. How does increased end diastolic volume lead to increased stroke volume? • Increased end-diastolic volume (the volume of blood in the heart's ventricles at the end of diastole, or the relaxation phase) leads to increased stroke volume (the amount of blood ejected from the heart with each beat). • When there is more blood in the ventricles at the end of diastole, the cardiac muscle fibers are stretched, following Starling's Law of the Heart. • This increased preload (end-diastolic volume) results in a more forceful contraction during systole (the contraction phase of the cardiac cycle). • The greater force of contraction leads to a larger volume of blood being ejected during the next beat, reinforcing the relationship between increased end-diastolic volume and increased stroke volume. Why is it important that right stroke volume = left stroke volume? Because if RSV was greater than LSV then blood would become congested in the pulmonary circulation and if LSV was greater than RSV, blood would become congested in the venous side of the systemic circulation. How are venous return and central venous pressure (CVP) involved in regulating enddiastolic volume (EDV) in the heart? • Venous return refers to blood flow from the body's systemic circulation back to the heart. • Venous return directly affects EDV: greater venous return leads to an increased EDV. • Central venous pressure (CVP) reflects right atrial filling pressure and is influenced by venous return. • An increase in venous return elevates CVP, promoting more effective right ventricle filling and increasing EDV. • A greater EDV, according to the Frank-Starling mechanism, results in a more forceful contraction and contributes to efficient cardiac output. What is the skeletal muscle pump, and how does it assist in venous return? • ◦ The skeletal muscle pump is a mechanism in the legs that aids venous return. ◦ During muscle contraction, veins are squeezed, and blood is propelled upward. ◦ Semilunar valves in the veins prevent backflow and ensure one-way blood flow toward the heart. ◦ Muscle relaxation allows veins to refill, and the process repeats, assisting in venous return to the heart. Explain the role of the respiratory pump in venous return to the right ventricle • The action of breathing helps to return blood to the heart • When we breathe in, the diaphragm flattens and presses down on the abdomen causing an increase in abdominal pressure • At the same time the chest wall expands which decreases pressure in the thorax • This pressure gradient sucks blood from the abdominal vena cava into the thoracic vena cava • This will deliv more blood to the right side of the heart and into the right ventricle • This increases filling of the heart and so increases EDV and by starling's law of the heart, the SV as well How does venous tone affect right ventricular filling? • 70% of our blood volume is contained within the venous circulation • Therefore, venous return can be increased by mobilising some of this stored blood • It is mobilised by vasoconstriction so that they cannot store as much blood • This moves blood back towards the heart and increases venous return. What mediates venous return? The sympathetic nervous system What is the most important factor that influences venous return? Gravity What happens when someone moves from the supine position to a standing position in terms of the cardiovascular system? You get a redistribution of blood due to gravity: Legs: - higher venous volume - higher venous pressure Thorax: - lower venous volume - lower venous pressure This leads to a fall in venous return and central venous pressure and as such, a decrease in EDV and SV Describe the distribution of blood in a supine position In the supine position the venous vessels will be approximately at the level of the heart: • this allows blood to be evenly distributed between all areas of the body from the head, down towards the feet • therefore, venous return is unaffected as central venous pressure can be maintained What is meant by preload? The stretch of myocardium How come atrial contraction, at rest, does not contribute much to end diastolic volume? Because atrial contraction contributes 5ml of blood to the ventricles at rest, with the majority being from passive filling How does atrial contraction play an important role in end diastolic volume during exercise? Sympathetic stimulation of atrial muscles increases the force of atrial contraction leading to a greater end diastolic volume and this maintains end diastolic volume during exercise because at higher heart rates seen in exercise the rapid passive filling phase of ventricles is shortened so it compensates for this How does heart rate influence EDV, particularly during exercise? • Our EDV is also influenced by our HR. • However, this is only at a high HR - when atrial contraction can’t compensate. • Diastole is approximately 2/3 of the cardiac cycle. • When HR increases the diastole time decreases. • But as most of the filling occurs during the rapid filling phase at beginning of atrial, systole, we can compensate for this shortening with and increased atrial contraction. • However, when HR > 180bpm, he passive filling plus the increase in atrial contraction is not sufficient to maintain EDV and therefore SV won’t be able to be maintained. • So, at HR > 180bpm, EDV will be reduced, so as ventricular muscle won’t be stretched as much, the SV will be reduced and so CO will be compromised. Where does the sympathetic nervous system originate? Thoracolumbar Where does the parasympathetic nervous system originate? Craniosacral What are the effects of sympathetic stimulation on the cardiovascular system? • increased heart rate and contractility (Beta-1 receptors) • vasoconstriction (alpha-1 and alpha-2 receptors) • some vasoconstriction (beta-2 receptors) • regulations of renin release for volume control (beta-1 receptor) Where are the perivascular nerves located in the vessels? The tunica externa/adventitia What allows for specific targeting of drugs? Receptor subtypes are located at relatively distinct cellular locations What adrenoreceptors are present in the vasculature? All the alpha subtypes and beta 2 Which adrenoreceptor is present in the heart? Beta 1 What are the effects of beta-adrenceptor activation on the heart? • increased heart rate by the positive chronotropic effect • increased contractility rate by the positive inotropic effect • increased automaticity (tendency to produce a spontaneous rhythm) • fast relaxation and recovery by the lusitropic effect What is the significance of beta-adrenceptor activation leading to increased automaticity in terms of resuss? Adrenaline administration When is exogenous adrenaline used in clinical practice? • the treatment of asystole, ventricular fibrillation and other severe arrhymias • anaphylaxis • commonly used in a mixture with local anesthetic because when it is injected locally it causes vasoconstriction What is adrenaline an agonist for? beta and alpho adrenceptors What is the difference between agonists and antagonists in pharmacology? • Agonists: ◦ Activate receptors when they bind. ◦ Mimic the action of endogenous ligands. ◦ Can be full or partial agonists. ◦ Induce a biological response. • Antagonists: ◦ Block or inhibit receptor activation. ◦ Compete with agonists for binding. ◦ Can be competitive or non-competitive antagonists. ◦ Prevent a biological response. What is the difference between competitive and non-competitive antagonists in pharmacology? • Competitive Antagonists: ◦ Compete for the same receptor binding site as agonists. ◦ Block receptor activation by directly competing with agonists. ◦ Reversible binding. • Non-competitive Antagonists: ◦ Bind to a different site on the receptor, causing conformational changes. ◦ Inhibit receptor activation without directly competing with agonists. ◦ May result in irreversible binding. What is dobutamine and what is it used for? beta-1 agonist and it is used to treat cardiogeneic shock by providing inotropic support What is phenylephrine and what is it used for? It is a alpha 1 adrenceptor agonist that leads to vasoconstriction and is used to treat nasal congestion. Because of this it is one of many potential ingredients in sudafed. What is midodrine and what is it used for? It is a prodrug for alpha 1 adrenceptor agonist and it leads to vasoconstriction with venoconstriction (due to increased capacitance) (this is more important) and it is used for postural hypotension in autonomic failure. Postural hypotension is also treated with a mineralocorticoid to increase circulating volume called fludrocortisone. What is droxidopa and what is it used for? It is a prodrug for noradrenaline (by analogy with dopa for dopamine) and is used for short-term treatment of postural hypotension in autonomic failure. What is postural hypotension? • Postural hypotension, or orthostatic hypotension, is a condition characterized by a significant drop in blood pressure when changing from lying down or sitting to a standing position. • Symptoms include dizziness, lightheadedness, and, in severe cases, fainting. • It can result from various causes, including dehydration, medication side effects, or underlying medical conditions. • Treatment depends on the underlying cause and may involve lifestyle changes, medication adjustments, and support garments What are the two alpha-2 adenoceptor agonists? 1. Clonidine - centrally-acting antihypertensive and it works by decreasing sympathetic drive 2. Brimonidine - direct transcutaneous vasoconstriction used in the treatment of rosacea What is doxazosin and what is it used for? It is an antagonist drug to the alpha-1 adrenceptor that causes vasodilation by opposing resting tone and it is used to treat hypertension and raynaud's syndrome. What is raynaud's syndrome? • Raynaud's Syndrome, also known as Raynaud's disease or Raynaud's phenomenon, is a medical condition that affects blood circulation, primarily in the fingers and toes. • It is characterized by episodes of vasospasm, where blood vessels in extremities constrict, causing reduced blood flow and color changes in the affected areas. • Common triggers include exposure to cold temperatures or emotional stress. • Raynaud's episodes can cause fingers or toes to turn white (pallor), then blue (cyanosis), and finally red (rubor) as blood flow returns. • While Raynaud's is not usually serious, it can be uncomfortable and may be associated with underlying medical conditions. What are beta-blockers and what are they used for? • Beta blockers are a class of medications that block the effects of stress hormones like adrenaline on beta receptors in the body. • They are commonly used to treat conditions such as high blood pressure (hypertension), angina, irregular heart rhythms (arrhythmias), and heart failure. • Beta blockers can also be prescribed to manage anxiety, migraines, and certain symptoms of hyperthyroidism. • By reducing the workload on the heart and blocking the effects of adrenaline, beta blockers help lower blood pressure, slow the heart rate, and improve heart function. What effects do beta blockers do? • negative chronotropic actions • negative inotropic actions • inhibit automaticity • therefore, decrease the work done by the heart Name drugs that block both alpha and beta adrenergic receptors. • Carvedilol and labetalol are examples of non-selective adrenergic blockers. • They block both alpha (alpha-1 and alpha-2) and beta (beta-1 and beta-2) adrenergic receptors. • Used to treat conditions like high blood pressure and heart-related issues. What is carvedilol? • Carvedilol is a non-selective adrenergic receptor blocker used as a medication. • It blocks both alpha-1, alpha-2, beta-1, and beta-2 adrenergic receptors. • Carvedilol is primarily prescribed to treat conditions such as congestive heart failure and high blood pressure. • Its action helps reduce heart workload and dilate blood vessels, leading to improved heart function and lower blood pressure. Where is the thoracic notch located? The thoracic inlet is located at the top of the thorax, at the base of the neck and the upper part of the chest. It is situated between the first thoracic vertebra (T1) at the back and the upper border of the sternum (breastbone) at the front. What are the anatomical boundaries of the thoracic inlet? 1. Posterior Boundary: The first thoracic vertebra (T1) forms the posterior boundary. 2. Anterior Boundary: The upper border of the sternum (manubrium) and the first pair of ribs (the first rib) form the anterior boundary. 3. Lateral Boundaries: The first pair of ribs and the first thoracic vertebrae on each side create the lateral boundaries. What structures pass through the thoracic inlet? Several important structures pass through or near the thoracic inlet, including the trachea, esophagus, major blood vessels (such as the brachiocephalic arteries and veins), and nerves. What is the clinical significance of the thoracic inlet? The thoracic inlet is a significant anatomical landmark for healthcare providers, especially when assessing and diagnosing conditions related to the neck, chest, and upper thorax. It is also relevant in understanding conditions like thoracic outlet syndrome, which can affect the neurovascular structures passing through this region. What structures form the costal margin? The costal margin is formed by the lower edges of the ribs. Specifically, the structures that contribute to the costal margin include the costal cartilages of the seventh, eighth, ninth, and tenth ribs, and to a lesser extent, the costal cartilage of the sixth rib. These cartilaginous extensions from the lower ribs create a curved, semi-rigid boundary along the lower edge of the ribcage and are important anatomical landmarks in clinical examinations and procedures. What structures can you palpate at the jugular notch? Tracheal cartilages Why do auscultation sites for heart sounds differ from valve surface projections? • Auscultation sites are selected for optimal sound transmission through chest tissues. • Heart sounds originate internally, not directly beneath the chest surface. • Healthcare providers place the stethoscope at specific sites to hear and assess cardiac sounds accurately. At what vertebral level is the manubriosternal joint located? T3 At what vertebral level is the xiphisternal joint? T9 What structures are you able to palpate at the jugular notch? The tracheal cartilages In which costal cartilage can you palpate the apex heart? 5th Why are peripheral pulses important in clinical assessment? • Peripheral pulses are essential for assessing cardiovascular health. • They serve as vital signs, indicating overall well-being. • Monitoring pulses helps diagnose and manage heart and vascular conditions. • Changes in pulse quality and symmetry may suggest pathology. • In emergencies, pulses aid rapid patient assessment. • They guide medication management and treatment effectiveness. Where is the axillary pulse located and what nearby/associated structures are there to it? • Location: Located in the axilla (armpit), near the shoulder. • Nearby/Associated Structures: Adjacent to the axillary artery, which supplies blood to the upper arm and shoulder. Where is the brachial pulse located and what nearby/associated structures are there to it? • Location: Found on the inner aspect of the upper arm, just below the shoulder and medial to the biceps muscle. • Nearby/Associated Structures: The brachial artery is located close to this pulse point. It is a major blood vessel that supplies the arm. Where is the radial pulse and what nearby/ associated structures are there to it? • Location: Located on the inner wrist, on the thumb side. It can be felt by placing fingers on the radial bone (radius). • Nearby/Associated Structures: The radial artery runs along the same path and is a major vessel supplying the forearm and hand. Where is the ulnar pulse located and what associated/nearby structures are there to it? • Location: Located on the inner wrist, on the little finger side. It can be felt by placing fingers on the ulnar bone (ulna). • Nearby/Associated Structures: The ulnar artery runs alongside the ulnar pulse point, supplying blood to the forearm and hand. Where is the femoral pulse located and what associated/nearby structures are there to it? • Location: Found in the groin area, near the crease where the thigh meets the pelvis. It can be located just below the inguinal ligament. • Nearby/Associated Structures: The femoral artery is located adjacent to the femoral pulse point and supplies blood to the thigh and lower extremities. Where is the popliteal pulse located and what associated/nearby structures are there to it? • Location: Located at the back of the knee joint, in the popliteal fossa, which is a small depression in the knee area. • Nearby/Associated Structures: The popliteal artery runs through the popliteal fossa, supplying blood to the lower leg. Where is the posterial tibial pulse located and what associated/nearby structures are there to it? • Location: Found on the inner side of the ankle, just behind and slightly below the medial malleolus (the prominent bone on the inner side of the ankle). • Nearby/Associated Structures: The posterior tibial artery runs along the same path and provides blood to the foot and calf muscles. Where is the dorsalis pedis located and what associated/nearby structures are there to it? • Location: Located on the top of the foot, just below the ankle joint, in line with the extension of the big toe. • Nearby/Associated Structures: The dorsalis pedis artery is adjacent to this pulse point and supplies blood to the toes and the top of the foot. How does the position of the head affect your ability to feel the carotid pulse lateral to the superior aspect of the thyroid cartilage? 1. Neutral Head Position: When the head is in a neutral, upright position, the carotid artery runs parallel to the neck. You can typically feel the carotid pulse easily by gently placing your fingers on the neck, just lateral (to the side) to the superior aspect of the thyroid cartilage. 2. Head Extension: If the head is extended backward, such as when tilting the head backward or looking up at the ceiling, the carotid artery may be more exposed and easier to palpate. This head position may provide even better access to the carotid pulse. 3. Head Flexion: Conversely, when the head is flexed forward, such as when looking downward or bringing the chin toward the chest, the carotid artery may be partially obscured by the surrounding structures, including the anterior neck muscles. In this position, it may be more challenging to feel the carotid pulse. Which vessel can be seen passing inferiorly across the sternocleidomastoid muscle and entering the subclavian vein? External jugular vein Where can the facial artery pulse be palpated and what are the nearby structures to it? 1. Location for Pulse Palpation: • The facial artery pulse can be palpated in the area of the lower jaw, slightly anterior to the masseter muscle, which is one of the chewing muscles. • Specifically, it is often felt at the inferior border of the mandible, near the angle of the jaw. 2. Nearby/Associated Structures: • Nearby structures include the mandible (lower jaw), the masseter muscle (one of the main muscles involved in chewing), and the buccinator muscle (a muscle of the cheek). • The facial artery itself, which carries oxygenated blood to the face, originates from the external carotid artery and runs a course through the face, supplying blood to various facial structures. Where can the superficial temporal pulse be palpated and what are the associated/nearby structures to it? 1. Location for Pulse Palpation: • The facial artery pulse can be palpated in the area of the lower jaw, slightly anterior to the masseter muscle, which is one of the chewing muscles. • Specifically, it is often felt at the inferior border of the mandible, near the angle of the jaw. 2. Nearby/Associated Structures: • Nearby structures include the mandible (lower jaw), the masseter muscle (one of the main muscles involved in chewing), and the buccinator muscle (a muscle of the cheek). • The facial artery itself, which carries oxygenated blood to the face, originates from the external carotid artery and runs a course through the face, supplying blood to various facial structures. Where is the aortic valve sound listened for? • Aortic Area (Second Right Intercostal Space): • Located in the second right intercostal space near the sternal border. • Best for listening to aortic valve sounds. Where is the pulmonary valve heart sound listened for? • Pulmonic Area (Second Left Intercostal Space): • Located in the second left intercostal space near the sternal border. • Ideal for auscultating pulmonic valve sounds. Where is the tricuspid heart sound listened for? • Tricuspid Area (Lower Left Sternal Border): • Located along the lower left sternal border. • Best for hearing tricuspid valve sounds. Where is the mitral valve sound listened for? • Located in the fifth left intercostal space at the midclavicular line (between the left nipple and the left midclavicular line). • Ideal for listening to mitral valve sounds, which are also known as the apex beat. Why do the heart valve sounds listened for in locations other than their surface projections? • Auscultation points provide optimal access to hear specific heart sounds. • Heart sounds are transmitted through complex pathways within the chest. • Sound waves travel through cardiac tissues, structures, and chest wall. • Clinical relevance guides the choice of auscultation points for accurate assessment. What is the effect of parasympathetic stimulation on blood vessels? • Limited vasodilation, because of limited innervation, despite widespread endothelial mAChrRs • Complicated by muscarinic receptor-induced release of NO from endothelial cells What is the effect of parasympathetic stimulation on the heart? • negative chronotropic action through action at the sinoatrial node • Slow AV node conduction • Little effect on myocardial contractility, but evidence for vagal modulation of ventricular rhythm How is acetylcholine released and modulated at nerve terminals? • Acetylcholine (ACh) is synthesized and stored in synaptic vesicles within the nerve terminal. • When an action potential reaches the nerve terminal, it depolarizes the membrane, opening voltage-gated calcium channels. • Calcium ions enter the nerve terminal, triggering fusion of ACh-containing vesicles with the presynaptic membrane and releasing ACh into the synaptic cleft. • ACh binds to postsynaptic receptors, leading to a depolarization of the postsynaptic membrane. • Acetylcholinesterase (AChE) in the synaptic cleft breaks down ACh to terminate the signal. • Modulation can occur through various mechanisms, such as autoreceptors, reuptake, or enzymatic degradation. What does modulation mean in neurotransmission, specifically at cholinergic synapses? • Modulation involves the regulation of neurotransmitter effects. • It influences the strength and duration of synaptic transmission. • At cholinergic synapses, modulation can occur through factors like receptor sensitivity, autoreceptors, reuptake, enzymatic degradation, and neuromodulators. What does cholinergic mean? It is a term used to describe anything related to or influenced by acetylcholine. Cholinergic neurones Cholinergic neurons are nerve cells that use acetylcholine as their primary neurotransmitter. These neurons release ACh to transmit signals to other neurons, muscles, or glands Cholinergic receptors Cholinergic receptors are specialized proteins on the surfaces of target cells (such as muscle cells or other neurons) that bind to acetylcholine. There are two main types of cholinergic receptors: nicotinic and muscarinic receptors. Nicotinic receptors One of the two main types of cholinergic receptor (the other being muscarinic). These are found at neuromuscular junctions and in the central and peripheral nervous system and they mediate fast excitatory responses. Muscarinic receptors One of the two main types of cholinergic receptors (the other being nicotinic receptors) that are found in various tissues, including smooth muscle, cardiac muscle, and certain glands. They mediate more varied responses compared to nicotinic receptors and can be excitaroy or inhibitory. Cholinergic efffects The effects of acetylcholine at cholinergic synapses vary depending on the specific receptor type, the location of the synapse, and the context. For example, cholinergic transmission is involved in muscle contractions, regulating heart rate, controlling secretions, and modulating neural circuits in the central nervous system. Cholinergic drugs Medications that affect the cholinergic system can be used for various purposes. For example, cholinergic agonists mimic the effects of acetylcholine and are used to treat conditions like myasthenia gravis, while cholinergic antagonists block the effects of ACh and can be used to treat conditions like overactive bladder. What are prodrugs and predrugs (precursor drugs) in pharmacology? • Prodrugs are inactive or less active compounds administered in an inactive form, converted to active drugs in the body. • Precursor drugs (pre-drugs) may refer to intermediate compounds in the synthesis of active pharmaceutical drugs but lack pharmacological activity. What does sympathetic drive refer to in physiology? • Sympathetic drive is the activation of the sympathetic nervous system. • It prepares the body for a "fight or flight" response to stress or challenges. • Involves physiological responses like increased heart rate, alertness, and redirection of blood flow. • Operates in balance with the parasympathetic nervous system. What does resting tone refer to in the context of neuroscience? • Resting tone in neuroscience describes the baseline level of muscle tension in skeletal muscles when the body is at rest. • It is important for maintaining posture, stability, and rapid responses to changes in body position. • Resting tone is regulated by the nervous system and plays a role in overall muscle function. What are the two main M2-mediated signalling pathways in the heart? 1. Direct G-protein mediated activation of K ACh 2. Inhibition of adenylate cyclase (ADC) - opposing PKA-mediated actions on a range of channels What is atropine and what are its cardiovascular uses? It is a muscarinic antagonist: • cardiac effects - block cardiac M 2 receptors • other effects (against all muscarinic receptors) - decreased secretions (mouth, airways, gut), bronchodilation, constipation, urinary retension, pupillary dilation, confusion/hallucinations What are the BNF approved uses of atropine? brachycardia following MI with hypotension, excessive bradycardia with betablocker use, intra-operative bradycardia What is the Uptake 1 block of noradrenaline? • Neuronal uptake of NAd • Mediated by NAT - noradrenaline transporter • is a secondary active transporter (requiring sodium and calcium) • blocked by cocaine and the tricyclic antidepressants • causes pro-arrhythmic effects of drugs such as cocaine How does cocaine's inhibition of norepinephrine reuptake lead to proarrhymic effects in the heart? • Cocaine inhibits the reuptake of norepinephrine. • This leads to excess norepinephrine at adrenergic receptors in the heart. • Excessive activation of adrenergic receptors disrupts normal cardiac rhythm. • Proarrhythmic effects, including ventricular tachycardia, can result, increasing the risk of life-threatening arrhythmias. How do monoamine oxidase inhibitors (MAOIs) interact with the cardiovascular system? • MAOIs can increase blood pressure by inhibiting the breakdown of norepinephrine. • They can lead to a hypertensive crisis when combined with tyramine-rich foods. • MAOIs may cause tachycardia, irregular heartbeats, and mydriasis. • They have the potential for serious cardiovascular side effects and drug interactions. What is a hypertensive crisis? • A hypertensive crisis is a severe, life-threatening increase in blood pressure. • It can lead to symptoms such as severe headache, chest pain, shortness of breath, and neurological symptoms. • Hypertensive crises can result from various causes, including uncontrolled high blood pressure or interactions with certain medications. • Immediate medical attention is necessary to prevent complications. What is the mechanism of action of methyldopa? • Methyldopa is converted to methylnorepinephrine. • Methylnorepinephrine stimulates central alpha-2 receptors in the brain. • This leads to reduced sympathetic nervous system activity. • Decreased norepinephrine release results in vasodilation and lowered blood pressure. Why is methyldopa used to treat hypertension in pregnancy? Because most of the other anti-hypertensives are contraindicated in pregnancy. What are the two ways that drugs can affect the heart? 1. Directly 2. Indirectly - by affecting the vasculature and changing blood volume, composition, and renal function and through this affect heart function What are arrhythmias? Disorders of heart rate or rhytm and they occur when there is abnormal generation or conduction of the electrical activity of the heart What are the three causes of arrythmia? • Pathological • Drug induced • Congenital What are the names of the different types of arrhythmias based on? Their origin and their effect How do arrhythmic drugs work? By altering the cardiac action potentials and/or ion currents which in turn affect heart rate/rhythm How do class 1 antiarrhythmic drugs work? By blocking sodium channels in cardiac cells What is the mechanism of action of Class 1a antiarrhythmic drugs? Class Ia drugs block sodium channels during depolarization, slowing the rate at which sodium enters cardiac cells and delaying repolarization. What is the mechanism of action of class 1b antiarrythmic drugs? Class Ib drugs block sodium channels, but they are particularly effective in damaged or ischemic cardiac tissue. They reduce the rate of sodium influx and can stabilize the cardiac membrane. What are the 9 protected characteristics as outlined in the equality act of 2010? 1. 2. 3. 4. 5. 6. 7. 8. 9. Age Disability Gender reassignment Pregnancy and maternity Race including colour, nationality, ethnic, or national origin Religion, belief, or lack of religion/belief Sex Sexual orientation Marriage and civil partnership What are human rights? Human rights are the basic rights and freedoms that belong to every person in the world from when they are born till they die. These rights are based on shared values like dignity, fairness, respect, and independence. The human rights act of 1988 is a British act that protects these human rights for its citizens. Why might we need drugs that affect rate/ rhythm? To treat disorders of rate and rhythm due to abnormal generation and conduction What is the most popular classification system for antiarrhythmic drugs? Vaughan Williams system Eg class 1 antiarrhytmic drugs Lidocaine, flecainide What is the target for lidocaine? Voltage gated sodium channels General mechanism of class 1 antiarrhythmic drugs Increasing refractory period of cardiac myocytes - decreased excitability Class 2 antiarrhythmic drug mechanism opposing sympathetic affect - these are beta blockers Metabolic/endocrine functions of the kidney • synthesis of hormones • vit d • erythropoietin • renin Homeostatic functions of the kidney • water/fluid • electrolytes • acid-base • blood pressure • elimination of waste • excretion of drugs and drug metabolities eg exogenous filtration markers inulin, 51Cr-EDTA What are the issues with using exogenous filtration markers? • require injection or infusion • require multiple sample collection • cumbersome and intrusive Problems with endogenous filtration markers • urinary clearance of creatinine • requires accurate timed urine collection and matched serum sample • cumbersome and error-prone When are techniques to measure glomerular filtration rate only used? Specific purposes when clinical decisions depend on accurate knowledge of GFR What is the first limitation of using serum creatine to measure GFR? there is a non-linear relationship between serum creatine and GFR, i.e. small changes in serum creatine value can mean large changes in GFR What is the second limitation of using serum creatine to measure GFR? • creatinine is an end product of muscle turnover so its production is preportional to muscle mass • it can also be derived from dietary meat or creatine supplements How do we quantify proteinuria? • Measure total amount - protein excretion in 24hr urine sample • Measure ratio to reference analyte, e.g. protein-creatinine ratio or albumincreatinine ratio How is CKD defined? Any abnormalities of kidney structure or function present for more than 3 months with implication for health Two features of CKD • irreversible loss of renal function (loss of nephrons) • progressive loss of renal failure if untreated Features of stage 5 CKD - end stage renal failure • insufficient renal function to sustain life or health • haemodialysis • kidney transplantation • death What GFR would be an indication of CKD? GFR < 60ml/min/1.73 m 2 What are the markers of kidney damage? • albuminuria • urine sediment abnormalities • electrolyte and other abnormalities due to tubular disorders • abnormalities detected by histology • structural abnormalities detected by imaging • history of kidney transplantation What albuminuria levels would suggest kidney disease? • AER - > or equal to 30mg/24 hrs • ACR > or equal to 30mg/g 0r 3mg/mmol Class II antiarrhythmic drug eg Metoprolol and other beta blockers How do class II antiarrhythmic drugs work? They decrease the sympathetic activity to the SAN Class 3 antiarrhythmic drug eg Amiodarone and sotalol What is the mechanism of action of class 3 antiarrhythmic drugs? These drugs are able to prolong action potentials by delaying repolarisation by blocking sodium channels Why can amiodarone lead to complications in individuals who have a thyroid function problem? Because iodine is part of its structure What is the mechanism of action of class 4 antiarrhythmic drugs? They decrease the rate of depolarisation, particularly at the AVN, slowing down conduction between the atria and ventricles by blocking L type calcium channels. eg class 4 antiarrhythmic drug Verapamil Why is verapamil described as being relatively cardio-selective? Because it principally has its action on cardiac myocytes and has no action in the vasculature What is the mechanism of action of adenosine? It acts on adenosine receptors on the SA/AV cells causing the potassium channels to open leading to hyperpolarisation meaning that the cell's action potential is further away from the threshold point at which an AP can be generated. This results in an increase in the refractory period thus slowing conduction through the SA and AV nodes What is adenosine, and what are its key functions? • Adenosine is a purine nucleoside composed of adenine and ribose. • Key Functions: ◦ Energy currency (ATP breakdown). ◦ Neuromodulator and neurotransmitter. ◦ Vasodilatory effect in cardiovascular regulation. ◦ Immunomodulation and anti-inflammatory properties. ◦ Role in cyclic adenosine monophosphate (cAMP) signaling. ◦ Medication use for cardiac testing and arrhythmia treatment. Why does caffeine have its effect on the heart? BECAUSE IT IS AN ANTAGONIST FOR ADENOSINE RECEPTORS. If you have too much caffiene you feel your heart is elevated leading to heart palpitations because the nodal cells are closer to threshold and are more likely to fire and so heart rate increases. What class of antiarrhythmic drugs is digoxin part of? It is non-classified What is digoxin's mechanism of action? Increases vagal activity increasing parasympathetic activity and as a consequence of this we get a decrease in the conduction rate in the AV node and a decrease in ventricular rate as well. What factors must be considered when deciding what antiarrhythmic drug to use? • Cause of arrhythmia (i.e. pathological, drug induced, congenital) • comorbidities - drug interactions - pharmacokinetics What are the general side effects of antiarrhythmic drugs? • All antiarrhythmic drugs affect ion balance across the cell membrane of cardiac myocytes. • Therefore, if we get drug doses wrong or use the wrong drug for a specific type of arrythmia, we could get to a situation where the drug actually causes an arrythmia. • In addition, as well as the drug interacting with ion channels that affect electrical activity they may affect other aspects of cardiomyocyte function that are dependent on ion action. • E.g. drugs can have a negative inotropy (force of contraction) action. Why do we need drugs that affect the force of contraction? Because there are some conditions where force of contraction is insufficient: • Anaphylaxis • Heart failure How is the force of contraction of cardiac myocytes normally determined? • Ca2+ is released from the SR. • This release is induced by calcium entry that occurs following depolarisation of cardiac myocyte membrane which allows for the opening of calcium selective channels. • The calcium in the cell can then promote the actin myosin interaction for contraction. • We can use this to understand how drugs affect influence force of contraction. What are the 3 classes of inotropic drugs? Sympathomimetics, cardic glycosides, phosphodiesterase inhibitors Positive inotropic drugs... ... increase intracellular calcium and therefore increase force of contraction allow Negative inotropic drugs... ... decrease intracellular calcium and decrease the force of contraction Is digoxin still extracted from plants? Yes as we cant relaibly synthesise it What is the mechanism of action of digoxin as a positive inotropic drug? Digoxin binds to and results in partial inhibition of Na + /K + ATPase (this is the protein responsible for maintaining the ion concentration gradient inside and outside cells). This leads to an accumulation of sodium inside the cell and an increase in intracellular sodium concentration: • if intracellular sodium levels rose the gradient for sodium between the outside and inside of the cell would diminish • therefore, this transporter would be less efficient at allowing sodium to enter and removing calcium (calcium extrusion is coupled with sodium import) • so calcium concentration increases intracellularly and an increase force of contraction Side effects of digoxin due to ionic disturbances • Side effects can occur due to ionic disturbances (changes in actions of Na/K ATPase & Na+/Ca2+ exchanger) ◦ The Membrane potential becomes more positive cells become closer to threshold value increase excitability more likely to fire AP lead to arrhythmia. ◦ Digoxin can cross the BBB and therefore impact neurones in CNS in a similar way. At a high toxic level, Digoxin can cause neurological disturbance ◦ Na/K ATPase is also found in the smooth muscle of GIT. So disturbing its function will disturb ion balance negatively impact SM function problem GIT function. Why can digoxin cause gyneacomastia? • Due to the structure of digoxin – it is very large. • When Digoxin is broken down you get a steroid ring that is similar to that found in oestrogen. • This molecule is able to fool oestrogen receptors breast growth • (This is quite rare) What subset of heart failure patients is digoxin used for? Those who have both contraction problems and an arrhythma Why do patients taking both diurects and digoxin need to be monitered closely? • Diuretics can be used in heart failure patients as well. • An individual who has indications that they should have both diuretic and Digoxin have to be monitored closely. • This is because Diuretics promote the loss of K+ from the body (hypokalaemia). • This decrease in K+ in the body promotes the relative Digoxin effect: ◦ This is because Digoxin usually binds to Na+/K+ ATPase at the same site as K+. ◦ I.e. they compete with each other. ◦ So, if there are fewer K+ ions in the cell, we have less competition easier for Digoxin molecules to bind to ATPase so we get an apparent increase in Digoxin effect despite having the same dose more likely to get adverse side effects mentioned earlier. What are phosphodiesterase? They're a group of enzymes that breakdown cAMP/cGMP (the secondary messenger molecules) Broadly how do phosphodiesterase inhibitors work? They inhibit the breakdown of phosphodiesterase enzymes and therefore increase the concentration of cAMP/cGMP within the cel of the drug targets. What is the specificity of phosphodiesterase inhibitors? • PDE enzymes are not uniformly found through the body. • There is a high distribution of PDE Type 3 found at the heart. • This means using specific PDE type 3 inhibitor, like Milrinone, means we can particularly adjust levels of cAMP within the cardiac myocyte. What are some adverse effects of PDE inhibitors? We may get increased excitability as PDE type 3 inhibition adjusts calcium levels in the cardiac myocytes resulting in arrhythmias. When would we use PDE type 3 inhibitors? • PDE type 3 inhibitors has very short heart life the amount of time drug is active for is short so patient has to be dosed regularly. • Some research has suggested chronic use actually decreases survival of patients with heart failure. • Therefore, these drugs are mainly used for patient going into acute heart failure (acute emergencies only) If inotropes are not very promising we can use ajust the other factros related to cardiac function? • We can decrease HR in order to adjust cardiac function. • This can allow everything to become balanced again of fluid in the lungs. fix congestive effect • Examples: ◦ Beta blockers can be used to reduce HR. ◦ Ivabradine: ▪ The IF channel in cardiac myocytes is important in determining the pace maker potential. ▪ Ivabradine blocks the IF channel – slows down HR by increasing time between beats. • Some drugs are used for heart failure that do not have a direct effect on heart cells ◦ Diuretics – decrease blood volume ◦ Vasodilators – increase systemic volume ◦ ACE inhibitors: Angiotensin converting enzyme inhibitors What does an ECG provide? A visual representation of the spread of electrical events through the heart P wave • Represents atrial depolarization • Duration (HR of 60bpm) = 0.10 s (100 ms) P-R interval • Begins at the start of the P wave and ends at the start of the QRS complex • It represents the time taken for the electrical activity to move between the atria and the ventricles. ◦ I.e. it reflects conduction through AV node. • Duration (HR 60bpm) = 0.12 - 0.20 s (120 - 200 ms) • A change in the P-R interval indicates delayed conduction through the AV node. • This can occur in isolation or co-exist with other blocks (e.g. second-degree AV block). T wave • Represents ventricular repolarisation • Duration (HR 60bpm) = 0.16 s (160 ms) R-R interval • Begins at the peak of one R wave and ends at the peak of the next R wave • You can use it to calculate HR What are the 12 standard ECG leads? • 3 standard limb leads • 3 augmented limb leads • 6 unipolar chest leads What do the 6 limb leads of the heart do? They look at the heart in a coronal plane How are the limb electrodes of an ECG arranged? Three electrodes, one each, to the right and left arm and left leg. What is the electrode attached to the right leg on an ECG? It is an earth electrode What are limb leads 1, 2, and 3? They are bipolar - they measure the voltage between 2 of the three limb electrodes What does limb lead 1 of an ECG represent? The voltage btween the right arm (negative pole) and the left arm (positive pole) and thus looks at the heart from the left What does limb lead 2 measure on an ECG? The voltage between the right arm (negative pole) and the left leg (positive pole) forming the inferior left view What does limb lead 3 of an ECG measure? The voltage between the left arm (negative pole) and the left leg (positive pole) looking at the heart from an inferior right angle What are the augmented limb leads on an ECG? aVR, aVL, and aVF and they are unipolar How do the augmented limb leads work? They use one limb electrode as the positive pole, and take the average of inputs from the other two as the zero reference How do augmented limb lead work on an ECG? They use one limb electrode as the positive pole, and take the average of inputs from the other two as the zero reference from the other two as the zero reference. ECG Chest leads key facts • The chest leads, view the heart in a transverse (HORIZONTAL) plane. • These are unipolar leads. • The corresponding chest electrodes serve as the positive poles. • The reference negative value is the same for all chest leads and is calculated as the average of inputs from the three limb electrodes. Describe the general mechanism of an ECG • The isoelectric line is the 'resting line'. • If a wave of electrical depolarization, moving through the heart, moves TOWARDS a lead it produces a POSITIVE deflection on the ECG (above the isoelectric line) • If a wave of electrical depolarization, moving through the heart, moves TOWARDS AWAY from a lead gives a NEGATIVE deflection on the ECG (below the isoelectric line) • The REVERSE is true for repolarization. • The magnitude of these deflections is greatest when the vector is moving directly toward the electrode (i.e. following the axis of the lead). • A vector that moves at right angles to the axis of a lead produces little deflection on the trace. What is Einthoven's Triangle It is an imaginary triangular formation of the three limb leads in a triangle used in electrocardiogram, formed by the two shoulders and the pubis. The shape forms an inverted equilateral triangle with the heart at the centre. What does constriction of arterioles to one organ do? Decreases flow to that organ, e.g. skin in cold conditions What does constriction of arterioles to multiple organs do? Increase total peripheral resistance and therefore increase arterial blood pressure. This is useful in situations where vasculure integrity is compromised, e.g. in haemorrhage or upon standing, because it helps maintain blood pressure. What is arteriole normal tone? The normal position of arterioles being slightly constricted at rest What results in the release of vasoactive substances by the endothelium that effect vascular muscle cells? Circulating hormones, paracrine hormones, shear stress, and hypoxia What is the most important vasoactive substance? Nitrous Oxide: • synthesised continuously by the enzyme NOS • It has a half life of less than 10s in vivo so its effects are very localised • its release leads to a fall in calcium levels in smooth muscle cells and thus causes vasodilation due to the relaxation of arteriole smooth muscle Describe the impact of nitrous oxide for coronary circulation • helps to increase coronary blood flow in exercise when cardiac activity and metabolism are increased • in coronary artery disease NO synthesis is reduced and this limits the ability of coronary flow to increase during exercise What is the most important endothelium derived vasoconstrictor? Endothelin Endothelin and pulmonary hypertension Treated with endothelin blockers What prostaglandin is a endothelium derived vasodilator? Prostacyclin What are many of the local factors responsible for regulation of blood flow? Metabolic by-products, e.g.: • adenosine - vasodilator • potassium - vasodilator • CO2 - vasodilator • Hydrogen ions - vasodilator How do local factors have an effect on regulating flow in tissues? Because resistance vessels, i.e. arterioles, are sensitive to these products and dilate when exposed to them What is reactive hyperaemia? Transient increase in flow seen after period of ischemia (no flow) usually due to arterial occulsion, e.g. the reactive hyperaemia seen in muscles after isotonic contraction during weight lifting. Why is reactive hyperaemia thought to occur? Due to the build up of metabolites during occlusion which are then washed out in the hyperaemia causing vasodilation allowing CO2 removal to increase - the longer the ischemia, the greater the hyperaemia What is active hyperaemia? Increase in flow due to increase in metabolic activity What is the increase in flow seen in active hyperaemia proportional to? The increase in metabolic activity What does active hyperaemia allow for? The increase in oxygen delivery when there is a increased oxygen demand Active hyperaemia potency example flow to skeletal muscle can increase 20-50 times dye to active hyperaemia during exercise What part of the nervous system innervates resistance arterioles? Sympathetic What are the exceptions to the fact there is little parasympathetic innervation to the blood vessels? Exocrine glands of the head and genitalia What is responsible for the vascular smooth muscle tonic vasomotor tone? Ongoing sympathetic nerve activity How does the sympathetic nervous system cause vasoconstriction? NA release from sympathetic fibres binding to alpha 1 adenoceptors on arterioles How does the sympathetic nervous system cause vasodilation? NA release from sympathetic nerve fibres binding to beta adenoceptors weakly and cause vasodilation. However, this is usually masked by alpha 1 stimulation What is the primary effect of circulating adrenaline? Increase HR and contractility The presence of what indicates circulating adrenaline is able to change the radius of arterioles? Alpha and beta adrenoceptors What is circulating adrenaline's vasoactive function? Vasodilation by having a higher affinity for B2 adrenoceptors on on the vessels. What is the vasoactive role of ADH? • major role in regulation of water retention when osmolality changes • helps maintain BP during haemorrhage • very high levels it causes vasoconstriction Vasoactive role of angiotension 2 Produced when the renal artery pressure falls and is a potent vasoconstrictor What happens during exercise in terms of changes in resistance? 1. During exercise sympathetic activity to blood vessels increases 2. Results in a reduction in blood flow to reproductive system, GIT, and kidneys 3. But, blood flow to muscles increases because locally produced metabolities are able to override the SNA and all for vasodilation, decreasing resistance, and increasing flow into the muscles How does the flow in an organ change due to the perfusion pressure? It will change preportionally to the perfusion pressure Myogenic mechanism of autoregulation of vascular radius • myogenic mechanisms are present in vascular smooth muscle such that when there is an increased intravascular pressure, smooth muscle will respond by contracting to restore the original diameter by vasoconstriction • this is due to vascular smooth muscle cells depolarising due to calcium entry • this is shown to varying degrees in different organs depending on how well said organs can tolerate a drop in blood flow in the face of a fall in pressure, e.g. coronary is very good at this but skin is not Autoregulation question - if the pressure was to fall you would get what in order to keep flow the same? Vasodilation What is meant by the autoregulatory range? This is the pressure range over which resistance vessels can constrict or dilate to compensate for a drop or increase in blood pressure to maintain the same blood flow. What happens as blood pressure drops below the autoregulatory range? Maximal vasodilation has been reached and so flow will start to fall What happens when blood pressure exceeds the autoregulatory range? Beyond this point any change in pressure will directly affect the blood flow and will lead to an increase in blood flow which could potentially be damaging. Compare the blood flow to the heart and brain to resting muscle and skin Heart and brain recieve more How does the level of oxygen consumption vary between tissues? Cardiac tissue has the highest compared to low in the skin What does a-VO2 mean? The max amount of oxygen that can be extracted by a tissue out of the oxygen delivered to it What is the max amount of oxygen that can be extracted from 100ml? 15ml How are vascular beds arranged? In parallel so that they all recieve the same arterial po2 What does the level of pO2 in the blood leaving a vascular bed depend on? The demands of that tissue, e.g. high demand means low pO2 leaving Where are the coronary arteries derived from? The aorta just distal to the aortic valve What do the coronary arteries lie on? The epicardic surfaces Broad course of the coronary veins Lie adjacent to the arteries and drain into the coronary sinus which then empties into the right atrium What are thebasian veins? Small, valve-less, veins that drain venous blood from the myocardium directly into any of the heart chambers, being most abundant in the right atrium, and least abundant in the left ventricle. They are also known as the smallest cardiac veins. What will thebasian veins cause in the systemic blood? Their presence causes a slight drop in oxygen content in the system blood Why is it that in the coronary circulation it is the regulation of flow that is responsible for matching oxygen supply to metabolic demand? Because the heart has a low capacity for anaerobic metabolism, with it recieving 5% of CO and extracting almost the maximum amount of oxygen possible at rest (very large a-VO2). Therefore, an increased demand for oxygen due to an increased heart rate must be met by a large flow increase that is roughly preportional to the increase in oxygen consumption. What are the two mechanisms that facilitate oxygen supply to cardiac cells? 1. The capillary density of myocardial tissue is very high with there being 1 capillary per myocyte 2. The transport of oxygen to cardiac cells is helped by the presence of myoglobin in cardiac myocytes Why does the high capillary density of myocardial tissue help oxygen transport? Because it crreates a large endothelium surface area for exchange and reduces diffusion distance - both facilitating delivery of oxygen and nutrients from capillaries to tissues as well as removal of metabolic by products How does the presence of myoglobin in cardiac myocytes facilitate oxygen transport? • While myoglobin can only bind 1 oxygen molecule, it has a higher affinity for oxygen compared to Hb • Therefore, in the capillaries of the coronary circulation Hb hands oxygen to myoglobin inside cardiac muscle cells • Myoglobin molecules inside the myocytes transfer the oxygen from one myoglobin molecule to the next speeding up the diffusion of oxygen through the muscle cell to the mitochondria What is coronary flow reserve? The difference between the resting level of flow and the max flow that can be obtained by dilating vessels By how much does the coronary flow reserve alow for flow to increase above resting levels? 5x What is the dominant form of regulation of the coronary flow? Metabolic/functional/active hyperaemia because it overrides autonomic control Describe how active hyperaemia of coronary flow works? 1. An increase in metabolic activity, a fall in coronary blood flow, or a fall in myocardial pO2 will all result in adenosine release 2. Adenosine is a potent vasodilator that acts by reducing intracellular calcium levels in the vascular smooth muscle cells 3. Extracellular potassium also rises as cardiac work increases and this may contribute to an intial increase in coronary perfusion but it is unlikely to mediate a sustained rise in coronary flow 4. When O2 demand exceeds O2 supply a rise in CO2 and acidosis may also lower vascular resistance to increase local oxygen supply 5. Endothelial derived vasodilators like nitrous oxide and prostacyclin also have a role. By how much does stenosis need to exceed to have a significant effect on flow? 60-70% Where does stenosis usually occur? In the large epicardiac arteries What does coronary artery disease cause that affects arteriole's ability to vasodilate? Atherosclerotic built up damages endothelial cells leading to a fall in nitrous oxide and prostacyclin production which are two important local factor vasodilators What type of angina is life threatening and why? Unstable angina because it indicates the danger of vessels becoming completely occluded and so often intervention including balloon angioplasty or implanting stents to open stenotic arteries or coronary bypass graft surgery is required What is angina? It is chest pain or discomfort caused by an imbalance between oxygen supply (decreased coronary blood flow) and oxygen demand (increased myocardial oxygen consumption), which leads to a decrease in the oxygen supply/demand ratio and myocardial hypoxia. What can the decreased blood flow in the coronary circulation seen in angina be caused by? Coronary artery vasospasm, fixed stenotic lesions (chronic vessel narrowing), or from a blood clot (thrombus) that incompletely (non-occlusive thrombus) or completely occludes a coronary artery (occlusive thrombus). Calcium-Channel Blockers (CCBs) and Vasospasm: • Mechanism: Inhibit entry of calcium ions into smooth muscle cells. • Effect: Prevent excessive smooth muscle contraction. • Result: Induce vasodilation of blood vessels. • Clinical Use: ◦ Treat conditions with vasospasm. ◦ Example: Used in coronary artery vasospasm. • Application: Alleviate vasospasm, improve blood flow to tissues. • Examples: Nifedipine, diltiazem. Vasospasm • Definition: Sudden, involuntary constriction or narrowing of a blood vessel. • Causes: ◦ Hypoxia/ischemia. ◦ Cold exposure. ◦ Emotional stress. ◦ Underlying medical conditions. ◦ Drug reactions. • Example: Coronary artery vasospasm can cause chest pain (angina). • Clinical Significance: Reduces blood flow to tissues. • Treatment: Address underlying causes; use medications like calciumchannel blockers to induce vasodilation. • Common Medications: Calcium-channel blockers (e.g., nifedipine) are used to prevent or relieve vasospasms. Nitrodilators and Vasospasm: • Mechanism: Nitrodilators release nitric oxide (NO). • NO Effects: ◦ Increases cGMP in smooth muscle cells. ◦ Induces vasodilation. • Prevents Vasospasm: ◦ Counters excessive smooth muscle contraction. • Clinical Use: ◦ Treatment of angina. ◦ Dilates coronary arteries in coronary artery vasospasm. • Common Medications: ◦ Nitroglycerin, isosorbide dinitrate. • Monitoring: ◦ Careful dosage and administration under healthcare supervision. How does vascular steal lead to angina? V ascular steal is a hemodynamic condition where multiple stenotic lesions can lead to a redistribution of flow within the major supply arteries of the heart under conditions of exercise or vasodilator therapy. Therefore, as blood flow increases in one region of the coronary vascular network, blood flow will reciprocally decrease in another region, leading to angina. What are the three types of angina? 1. Variant 2. Stable 3. Unstable Compare the three types of angina What is stable angina? The most common type of angina, caused by a narrowing of the coronary arteries that reduces blood flow to the heart. It typically occurs during physical activity or emotional stress and goes away with rest or medication. How does the narrowing of the coronary arteries cause angina in stable angina? When a coronary artery narrows beyond a critical value known as critical stenosis, the myocardial tissue perfused by the artery will not recieve adequate blood flow because the coronary flow reserve decreases resulting in the tissue becoming ischaemic and hypoxic, particularly during times of increased oxygen demand like physical exertion. How is stable angina treated? Lifestyle changes, such as diet and exercise, and medications, such as nitroglycerin (nitrodilators), beta-blockers, and calcium-channel blockers, are used to treat stable angina What is unstable angina? A more serious type of angina that can occur at rest or with less exertion than stable angina. It is a sign that the coronary arteries are narrowing or becoming blocked, which can lead to a heart attack. How is unstable angina treated? With drugs that reduce oxygen demand (i.e. beta blockers, calcium channel blockers, and nitrodilators) and, most importantly, drugs that inhibit thrombus formation (e.g. anti-platelet drugs and aspirin) How does unstable angina work? It is caused by the transient formation and dissolution of thrombosis within a coronary artery. These clots often form in response to plaque rupture in atherosclerotic coronary arteries, but it may also form because diseased coronary endothelium is unable to produce NO and prostacyclin that inhibits platelet aggregation and clot formation. When the clot forms, coronary flow is reduced, leading to supply ischaemia. How can unstable angina lead to acute myocardial infarction? Unstable angina is characterised by the transient formation and dissolution of thrombosis and if one of these thrombosis completely occludes the coronary artery for a sufficient amount of time, the myocardium supplied by the vessel will become infarcted - aka a acute myocardial infaction has occured. How is an acute myocardial infarction different from a myocardial infarction? An acute myocardial infarction is one that comes on suddenly due a sudden blockage of a coronary artery that causes damage to the myocardium, whereas a myocardial infarction is a broader term that encompasses both an AMI and other more slower onset forms. In effect, myocardial infarction is an umbrella term and AMI is a sub-type. What is variant angina? A type of angina that is caused by spasms in the coronary arteries, which temporarily reduce blood flow to the heart. It typically occurs at rest and is often associated with emotional stress, exposure to cold temperatures, or smoking. How is variant angina treated? Nitrodilators and calcium-channel blockers How does vasospasm cause variant angina? Because it temporarily reduces coronary blood flow, producing ischaemia by reducing blood supply, thereby decreasing the oxygen/demand ratio. How does enhanced sympathetic activity precipitate vasospastic angina (variant angina)? Enhanced sympathetic activity, especially when coupled with with a dysfunctional coronary vascular endothelium (i.e. reduced endothelial production of the vasodilators nitric oxide and prostacyclin) can precipitate vasospastic angina. What is demand ischaemia? A type of ischaemia that occurs when the heart's demand for oxygen exceeds its supply. Critical stenosis • Definition: Critical stenosis refers to a severe narrowing or constriction of a blood vessel, often due to atherosclerosis or other pathological processes. • Significance: This level of stenosis significantly reduces blood flow through the affected vessel, compromising oxygen and nutrient delivery to downstream tissues. • Clinical Implications: Critical stenosis is a key factor in conditions like coronary artery disease, where reduced blood flow to the heart muscle can lead to ischemia and potentially trigger heart-related events. • Diagnostic Tools: Detected through imaging studies (e.g., angiography) that visualize and assess the degree of narrowing. • Treatment: Interventions, such as angioplasty or stent placement, may be necessary to restore proper blood flow and prevent complications. • Monitoring: Regular assessment is crucial to manage and prevent complications associated with critical stenosis. What is stenosis? The narrowing of an artery or other passage in the body Neuronal hypoxia longer than ... leads to neuronal damage 4 mins How quickly does syncope (fainting) occur after cerebral ischaemia? seconds How does local cerebral blood flow alter? Metabolic/functional hyperaemia Grey matter vs white matter - which recieves the majority of blood flow to the brain? Grey matter as it contains the neuronal cell bodies What is the blood flow to the brain through? 2 internal carotid and 2 vertebral arteries What anastomoses to form the circle of willis? The 2 internal carotid and 2 vertebral arteries What is the significance of the circle of willis being an anastomosis? If one of the source arteries (i.e. the internal carotid or vertebral) develops a stenosis or is obstructed the other source arteries can provide alternative flow it is protective of the brain blood flow What are the pial arteries Definition: Arteries associated with the pia mater, the innermost layer of the meninges covering the brain and spinal cord. Pial arteries play a crucial role in supplying oxygenated blood to the nervous tissue. 1. Location: Found within the pia mater, the innermost layer of the meninges. 2. Function: Responsible for supplying oxygenated blood to the brain and spinal cord. 3. Anatomy: Pial arteries branch out into smaller vessels, forming an intricate network to ensure proper blood supply to different regions of the nervous system. 4. Clinical Significance: Disruptions in blood supply to the brain through pial arteries can lead to serious conditions such as strokes or other vascular disorders. Do the capillary networks of the brain have high or low resistance? Relatively high resistance What is different about the brain capillaries compared to the systemic circulation? In the systemic circulation capillaries are leaky due to the presence of fenestrations which allow for substances to cross the capillary wall. However, these fenestrations are not present in the capillary walls present in the brain because of the blood brain barrier - instead the edges of adjacent endothelial cells are joined together and completely sealed via tight junctions, preventing bulk flow and diffusion of water and ions that is seen in systemic circulation. What is the blood brain barrier? Definition: A selective semipermeable membrane barrier that separates the bloodstream from the brain's extracellular fluid, formed by tight junctions between endothelial cells in brain capillaries. Key Points: 1. Function: Protects the brain by restricting the passage of potentially harmful substances from the bloodstream. 2. Structure: Tight junctions between endothelial cells create a physical barrier, selectively allowing essential nutrients while blocking larger or hydrophilic molecules. 3. Role of Astrocytes: Astrocytes contribute to BBB regulation, releasing chemical signals that influence barrier permeability. 4. Significance: Essential for maintaining the optimal neural environment; poses challenges for drug delivery to the brain, prompting ongoing research for improved methods. What is the significance of the blood brain barrier for substance transport? All substances entering or leaving the brain must pass through the 2 plasma membranes and the cytoplasm of the endothelial cells rather than moving between cells: • Like all capillaries, the cerebral capillariers are permeable to lypophilic solutes like oxygen, carbon dioxide, alcohol, nicotine, and caffeine. • Glucose and plasma proteins move through cells via plasma proteins • Carrier proteins move surplus molecules out preventing a build-up of anything that could interfere with brain function: - when potassium in the interstitium increases due to neuronal activity it is pumped out via the sodium/potassium ATPase pump, helping to regulate cerebral interstitium potassium concentration - this is the reason why cerebral endothelium cells have 5-6x as many mitochondria than muscle cells How many more mitochondria do do cerebral capillary endothelium have compared to muscle endothelium? 5-6 times more How is the blood flow to the brain safeguarded? Through the regulation of peripheral resistance to other vascular beds - when neccessary, the perfusion of peripheral organs (except the heart) is sacrificed through vasoconstriction to maintain arterial pressure and therefore cerebral flow. How does autoregulation safeguard cerebral blood flow? If the blood pressure falls, cerebral resistance vessels dilate to maintain cerebral flow due to the release of local factors like NO and prostacyclin. They are able to compensate for pressure changes between 60-150 mmHg. Hypotension below 50-60 mmHg leads to the failure of autoregulation and decreased blood flow, and so decreased oxygen perfusion, and so mental confusion and syncope. How are cerebral vessels responsive to CO2? Very: 1. Hypercapnia induces vasodilating of cerebral vessels which is useful during suffocation to maintain oxygen delivery: • small pial arteries dilate more than larger cerebral arteries in response to CO2 • the vasodilation is partly mediated by endothelial NO and is perhaps aided by a fall in myocyte pH 2. Hypocapnia invokes vasoconstriction of cerebral vessels: • an arterial CO2 level of 2pka will half total cerebral flow • this is responsible for the dizziness experienced in hypocapnia Hypercapnia High CO2 levels in the blood (PaCO2 >5kpa) Hypocapnia Low CO2 levels in the blood (PaCO2 <5kpa) In comparison to their responsiveness to CO2, how does the responsiveness of cerebral vessels compare for O2? Less responsive: - a halving of normal PO2 is unlikely to affect cerebral flow - severe hypoxia will lead to vasodilation in cerebral circulation via adenosine, potassium, and NO Why does severe hypoxia often lead to a masking of the expected vasodilation in cerebral vessels? Because severe hypoxia invokes hyperventilation, which will, in turn, lead to a fall in PCO2, and therefore cerebral vasoconstriction will occur and will counteract the vasodilation seen from the severe hypoxic response. This is because cerebral vessels are far more responsive to PCO2 than PO2. What is meant by neuronal activity-evoked functional hyperaemia? While total cerebral blood flow is relatively constant due to autoregulation, regional changes in blood distribution occur in response to changing patterns of neuronal activity with flow shifting towards areas of increased neuronal activity, i.e. blood distribution will be different in a person sleeping to a person studying. What factors are important in coupling tissue metabolism to local cerebral flow? • Increased interstitial potassium ion concentration • adenosine • neuronal NO • metabolites released from astrocytes during increased neuronal activity • CO2 level How does increased interstitial [K+] aid in coupling cerebral tissue metabolism to local flow? • neuronal activity increases potassium ion permeability of the membranes • the extracellular potassium concentration can increase 3x the basal level • potassium ions are a potent vasodilator so you see increased flow How does adenosine aid in coupling cerebral tissue metabolism to local flow? Because it is released when metabolism increases and is a vasodilator How does neuronal nitric oxide aid in coupling cerebral tissue metabolism to local flow? Neuronal activity causes the release of neuronally derived nitric oxide and it is a vasodilator so leads to vasodilation which leads to increased blood flow by decreased resistance How do metabolites released by astrocytes during increased neuronal activity aid in coupling cerebral tissue metabolism to local flow? The astrocyte end foot processes envelope blood vessels in the brain and they send signals to the arterial/arteriole smooth muscle cells which allows for them to control vessel diameter How does PCO2 aid in coupling cerebral tissue metabolism to local flow? Increased metabolism increases CO2 production which will cause vasodilation - > minimal effect How is it that the intracellular space is a K+ reserve? Becasue it exists in the intracellular space at a higher concentration than the extracellular space (140mM vs 4mM) combined with the much larger volume of the intracellular space compared to the extracellular space (14l vs 28l) Where will any additional potassium in the extracellular space go? It will be taken up into the cell - any potassium we ingest is moved into cells quickly by an active process What is the maximal increase to cerebral resistance vessels resistance that sympathetic stimulation can exert, and how does this compare to the skeletal muscle? 20-30% - compared to 500% Baroreceptors have little influence on cerebral flow, otherwse every time you stood up or exercised... we would compromise cerebral blood flow by increasing sympathetic stimulation What are baroreceptors? Baroreceptors are pressure-sensitive receptors located in blood vessels, particularly in the carotid sinus and aortic arch. They monitor changes in blood pressure by detecting the stretch of arterial walls. When blood pressure increases, baroreceptors signal the brain's cardiovascular center, leading to adjustments such as decreased heart rate and vasodilation to lower pressure. Conversely, if blood pressure drops, the response includes increased heart rate and vasoconstriction. Baroreceptors play a vital role in maintaining blood pressure within a normal range and are part of the autonomic nervous system. What is the carotid body? The carotid body is a cluster of chemoreceptor cells located near the common carotid arteries. It monitors blood oxygen, carbon dioxide, and pH levels. When hypoxia, hypercapnia, or acidosis occurs, the carotid body stimulates respiratory centers in the brainstem to adjust breathing. This helps maintain proper blood gas levels and supports overall respiratory regulation. What are the three intracranial constituents? Tissue, blood, and CSF An increase in intracranial pressure comes about by what? Either one of the three constituents of the intracranium increasing in volume An increase in the volume of one of the three constituents of the intracranium means that? If tissue increases, then blood and CSF would need to decrease and vice versa What is raised ICP commonly caused by? Intracranial bleeding, cerebral oedema, and tumour growth What does high ICP result in? Collapsed veins and a decrease in the effective cerebral perfusion pressure leading to a reduced blood flow How do you calculate cerebral perfusion pressure? ABP-ICP What is the normal range of ICP? 5-15 mmHg When there is an increase in ICP past ... combined with ... then cerebral blood flow can be significantly reduced 20 mmHg and systemc hypotension What is a transient ischaemic attack? It is a temporary reduction in flow to a cerebral tissue that can last a few minutes to hours. What is an ischaemic stroke? An ischaemic stroke occurs when there is a total interruption in cerebral flow due to atherosclerosis or blood clot in an extracerebral artery What is a haemorrhagic stroke? • Definition: A type of stroke caused by bleeding in or around the brain. • Types: ◦ Intracerebral Hemorrhage (ICH): Bleeding within the brain tissue. ◦ Subarachnoid Hemorrhage (SAH): Bleeding into the space between the brain and its covering. • Symptoms: Sudden severe headaches, weakness, numbness, difficulty speaking, and visual disturbances. • Causes: High blood pressure, cerebral aneurysms, arteriovenous malformations. • Treatment: May involve surgery to repair bleeding vessels or other interventions. • Importance of Prompt Medical Attention: Critical for minimizing damage and improving outcomes. What is a thrombus? A thrombus is a blood clot that forms and remains attached to the vascular wall at the site of its formation. It can partially or completely block blood flow, depending on its size and location. Thrombi are often associated with conditions such as atherosclerosis or damage to blood vessel walls. What is an embolus? An embolus is a detached, moving blood clot or other foreign material that travels through the bloodstream. It can potentially lodge in a smaller blood vessel and cause an obstruction, leading to conditions such as pulmonary embolism (if it lodges in the lungs) or stroke (if it reaches the brain). What is an embolism? An embolism is the blockage of a blood vessel by an embolus. This blockage can impede blood flow and cause damage to tissues supplied by the affected vessel. Depending on where the embolism lodges, it can lead to various medical emergencies. What is a DVT? Deep vein thrombosis refers to the formation of a blood clot (thrombus) within a deep vein, typically in the legs. If a clot from a DVT breaks loose and travels to the lungs, it can cause a pulmonary embolism. What is pulmonary embolism? A pulmonary embolism occurs when an embolus, usually from a deep vein thrombosis, travels to the pulmonary arteries in the lungs and blocks blood flow. This condition can be life-threatening and requires prompt medical attention. What is thrombophlebitis? Thrombophlebitis is the inflammation of a vein accompanied by the formation of a thrombus. It often occurs in the legs and can cause pain, swelling, and redness at the affected site. Infarction obstruction of the blood supply to an organ or region of tissue, typically by a thrombus or embolus , causing local death of the tissue. What is the main function of cutaneous circulation? Maintanance of constant body temperatrue - thermoregulation Describe the range in blood flow to the cutaneous circulation • When ambient temperature falls below thermoneutral zone, cutaneous blood flow can fall as low as 1ml per minute per 100g of tissue. • A rise in core temperature can increase flow to skin to 200x that level. What important feature of the cutaneous circulation is responsible for its thermoregulatory ability? The arterio-venous anastomoses - blood can bypass the capillaries in the epidermis and flow straight into venous plexi (allowing for a rapid expansion in cutaneous blood volume - remember veins are storage vessels because they can dilate so much). Where are A-V anastomoses particularly prominent? The skin of the nose, lips, ears, toes, and especially fingertips - why these go red when hot Describe the cutaneous thermoregulatory response to increased body temperature Increased body temperature causes the arterioles of the skin to vasodilate due to the withdrawal of sympathetic tone, including the arterioles of the arterio-venous anastomoses. Therefore, blood flow into the cutaneous circulation increases massively, accomodated by the arterio-venous plexi vasodilation increasing flow into the venous plexi, which are able to stretch to hold this increased blood volume (veins are the compliance vessels). This massive increase in cutaneous flow accomodated by the A-V anastomoses and the venous plexi provide a large surface area for heat exchange between the body (blood) and the environment, lowering body temperature. This affect is combined with an increase in sweat production stimulated by the hypothalamus through cholinergic fibres innervating sweat glands. This is significant for two reasons: 1. Sweat evaporation speeds up energy (heat) exchange with the environment 2. Sweat enhanced vasodilation of the vessels because it contains an enzyme that acts on tissues to stimulate bradykinin release. This bradykinin acts locally on through paracrine signalling to relax smooth muscle cells and enhance vasodilation, possibly through the formation of NO How is blood pressure maintained in response to the vasodilation seen in the cutaneous circulation after an increase in body temperature? • The vasodilation seen in response to increase temperature has consequences for whole CVS: ◦ Vasodilation results in fall in TPR and this leads to fall in Blood pressure if there is no accompanying increase in CO. ◦ Via the baroreceptor reflex, CO is increased predominantly by increasing HR. ◦ Increasing HR is brought about by increase in SNS activity and decrease in vagal activity to SA node and by the direct effect of increase temperature on cells of SA node. ◦ Therefore, a tachycardia of 10bpm per degrees increase in temperature, ensure BP is maintained. How can an increase in body temperature result in syncope? Because increases in temperature lead to vasodilation of the resistance vessels of the cutaneous circulation due to a withdrawal in sympathetic tone to these vessels in the cutaneous circulation. This happens because of the thermoregulatory role of the skin - increasing blood flow to the skin, and therefore the blood volume in the skin (A-V anastomoses) enhances heat exchange and lowers temperature. However, due to this vasodilation, there is a fall in total peripheral resistance, and therefore a fall in blood pressure unless there is an increase in cardiac output. Therefore, an inability to increase cardiac output sufficiently can result in falls in BP being too great to maintain adequate cerebral perfusion and therefore syncope occurs to restore cerebral blood flow. Why does syncope occur when there is a decreased blood flow to the brain? Because syncope, a sudden temporary loss of consciousness, results in the person falling down into a horizontal position. Being in a horizontal position means that cerebral perfusion can be restored because the circulatory system no longer has to work against gravity and allows for the blood pooled in the legs due to gravity to return. Therefore, venous return is increased, preload is increased, and cardiac output is increased which ensures adequate cerebral perfusion. How does the cutaneous circulation respond to decreased body temperature? • If temperature drops there is an increase in sympathetic activity to the cutaneous vessels and an increase NA release, leading to vasoconstriction of cutaneous arterioles. • There is also an increase in sympathetic activity to A-V anastomosis, this results in their constriction prevent blood flow through to vein. • These changes increase resistance to blood flow and divert blood to low resistance vessels in the interior of body. • This minimises heat loss by keeping blood away from surface, blood diverted to deep veins that lie beneath insulating fat. What and why does paradoxical cold vasodilation occur? • With prolonged exposure to cold the vasoconstriction in the cutaneous circulation changes to a paradoxical cold vasodilation. • This gives the skin a red appearance. • The example shown here is taken form human studies where blood flow to calve is measured by a laser Doppler flux metre. • The dilation is thought to be due to paralysis of noradrenergic neurotransmission is response to cold and the release of vasodilators such as prostacyclin • The redness of the blood is largely due to the increase in affinity of Hb for O2 as reflected by leftward shift of o2 dissociation curve when temperature is reduced. • Cold induced vasodilation is thought to prevent cold weather injury to tissues. Describe the action of vasodilators Vasodilators have an indirect action on the heart function by having a direct action on the smooth muscle cells of the vasculature What are the two different ways that disease states can involve the vasculature? • Systemically • Locally What are the three ways vascular tone is maintained? • Autonomic innervation - systemic factor • Circulating hormones - systemic factor • Local factors - affect vasculature in their immediate area What are the two processes that can be targeted by pharmocological vasodilators? • Indirectly by targeting circulating vasoconstrictors • Directly by targeting the processes going on in the smooth muscle cell Two divisions of vasodilator drugs Direct and indirect Three ways that vasodilator drugs have an indirect action? 1. Blocking the autonomic nervous system - sympathetic blockage via alpha-1 adrenoceptor antagonists -> block the vasoconstricting action of noradrenaline and will result in vasodilation 2. Renin-angiotension-aldosterone system (RAAS) - angiotension 2 is a potent vasoconstrictor, therefore, blocking its action using antagonists will cause vasodilation 3. Enothelins - circulating factors that cause vasoconstriction -> targeting these is an active area of research What are the two sources of calcium in vascular smooth muscle cells? • Intracellular store release • Entry from extracellular sources via voltage gated calcium channels What are the three main targets for indirect vasodilator drugs? • VC calcium channesl • Membrane potential - because calcium ion channels are voltage gated, anything that impacts membrane potential will impact these channels and so impact intracellular calcium levels • cGMP - intracellular messenger that is an important determinant of smooth muscle action that affects how long calcium channels are opne and, through intermediates, affect myosin acting interaction What are the three classes of drugs that treat angina? 1. Class A - not vasodilators, includes beta-blockers and ivadradine 2. Class B - Organic Nitrates - Vasodilators 3. Class C - Calcium channel blockers - Vasodilators What are the two main class A drugs used to treat angina? Beta-blockers and ivabradine How do beta-blockers treat angina? beta-blockers alleviate angina symptoms by decreasing the heart rate, reducing the force of cardiac contraction, lowering blood pressure, and improving the balance between oxygen supply and demand in the heart muscle What is ivabradine and what is it used for? It inhibits IF ion channel which is responsible for the pacemaker potential that sets heart rate. Its inhibition will reduce heart rate and decrease the cardiac work. It is used to treat angina. How do nitrate drugs like GTN sprays work? These drugs are a class of vasodilators and they work by: • they contain nitrogen atoms and so when broken down in the body they create NO • NO is a physiological signalling molecule involved in smooth muscle control • NO through its actions on the enzyme guanylate cyclase will result in an increase in cGMP • Increasing cGMP promotes relaxation of the smooth muscle cells = vasodilation • this occurs due interference with myosin-actin interaction and the enhancement of calcium efflux How does increasing cGMP levels affect calcium levels in vascular smooth muscle cells, and what does this result in? • Increased cGMP activates PKG. • PKG phosphorylates proteins, inhibiting calcium entry. • Calcium efflux is enhanced. • Result: Smooth muscle relaxation due to decreased intracellular calcium levels. • Important in vasodilation and used in medications like nitroglycerin and PDE5 inhibitors Therefore intracellular levels decrease and vasodilation occurs due to smooth muscle cell relaxation What is cGMP? Prominent second messenger molecule What are second messengers, and what is their role in cellular signaling? • Definition: Second messengers are molecules that relay signals from cell surface receptors to the cell interior. • Examples: ◦ Cyclic AMP (cAMP) activates protein kinase A (PKA). ◦ Cyclic GMP (cGMP) regulates smooth muscle relaxation. ◦ Inositol trisphosphate (IP3) releases calcium; diacylglycerol (DAG) activates protein kinase C (PKC). ◦ Calcium (Ca2+) influences various cellular processes. ◦ Nitric oxide (NO) activates guanylate cyclase, producing cGMP. • Role: Essential for signal transduction, allowing cells to respond to external stimuli and regulate physiological processes. What is shear stress in the cardiovascular system? • Definition: Shear stress is the force per unit area parallel to the vessel wall generated by blood flow. • Endothelial Response: ◦ Influences endothelial function and integrity. ◦ Important for the release of nitric oxide and signaling molecules. • Effects: ◦ Beneficial in moderate and regular patterns. ◦ Altered patterns, like low shear stress, can contribute to atherosclerosis. • Measurement: Typically measured in units of force per unit area (e.g., dyn/ cm² or pascal, Pa). Define physiological Refers to anything related to the normal functioning of living organisms and their parts What is afterload? • Definition: Afterload is the resistance the left ventricle must overcome during systole to eject blood into the aorta. • Determinants: ◦ Influenced by arterial tone and systemic resistance. ◦ Arterial blood pressure is a major factor. • Clinical Implications: ◦ High afterload increases the heart's workload. ◦ Conditions like hypertension contribute to elevated afterload. • Impact: ◦ Affects cardiac output and can lead to left ventricular hypertrophy with chronic elevation. What is the mechanism of action that explains how GTN relieves angina? • GTN affects the systemic vessels and the venous vesses are affected more than the arterial vessels • Due to it affecting venous vessels and venous vessels being the storage vessels of the CVS, we increase blood storage in veins, and thus decrease venous return greatly • This in turn reduces cardiac work via starling's law and therefore the oxygen demand of the coronary tissue is greatly reduced • There is a significant enough reduction in cardiac work and thus oxygen demand for the coronary blood supply to maintain sufficient oxygenation of cardiac myocytes Is hypotension an unwanted effect of GTN sprays? Yes, due to its systemic effects GTN can cause excessive vasodilation which can lead to hypotension leading to syncope -> one of the responses to this hypotension is reflex tachycardia via the baroreceptor reflex -> this is bad becaue patients who require GTN have compromised coronary blood flow, and GTN sprays are meant to decrease cardiac work and therefore cardiac myocyte oxygen demand but here it is having the opposite effect How can GTN cause a headache? By causing excessive vasodilation of the blood vessels supplying the head How does GTN affect the GIT? By relaxing smooth muscle in the GIT it can cause a reduction of motility Why can't GTN be administered orally? Because upon being absorbed from the GIT, it is completely metabolised through first pass metabolism in the liver - so this way it is removed before it can even have its effect. What are the two ways that GTN is administered? Sublingually and transdermally Why is GTN often administered sublingually? Because it cannot be administered orally due to it being removed completely in first pass metabolism and so must be administered in a way that allows it to exerts its effect before being metabolised in the liver, and the highly vascularised underside of the tongue provides the perfect place for this. Why do people with angina often only use GTN sublingually for immediate and short-term symptomatic relief but not long term relief? Because it is metabolised by the body so quickly do to it not surviving first pass metabolism Why is the option of transdermal administration of GTN important? Because GTN does not survive first pass metabolism it cannot be administered orally and nor does it stick around long enough to have long acting effects when administered sublingually. Transdermal administration allows the drug to be constantly diffused into the body at low levels to prevent angina attack. What organic nitrate medication can be administered orally? Isosorbide mono/dinitrate - works in the same way as GTN but with different pharmacokinetics that mean it can be given orally and have a longer duration of action How is the development of tolerance a problem for GTN treatment? • Physiological tolerance - the blood vessels have multiple factors that control their state and GTN only acts on one of these pathways so over time the other factors can adapt to counteract GTN action • Pharmacological tolerance - there is a fundemental change in the way the drug interacts with the target with constant exposure of the target to the drug that can mean the system stops responding to the drug What is the mechanism of action of vasodilators that target calcium blockers (class 3 for angina treatment)? • Blocking voltage gated calcium channels reduces the efflux of calcium into the smooth muscle cells and so reduces the intracellular concentration of calcium and therefore the level of contraction (remember that calcium can also be sourced intracellularly from intracellular calcium stores) What is an example of a family of drugs that are VG calcium channel blocking vasodilators? DHPs (dihydropyridines) which act on the vasculature, e.g. amlodipine Why is selectivity important for VG calcium channel blocking drugs? VG calcium channels are instrumental in determing contractility of all smoot muscle cells and as such are found throughout the CVS. However, blocking VG calcium channels in the SM of the vessels and then blocking them in cardiac myocytes exerts different affects. Therefore, drugs that selectively target either VG calcium channels on cardiac myocytes or SM of blood vessels is important for targeting treatment, e.g. verapamil is selective for cardiac myocyte VG calcium channels and therefore is used to treat arrythmia, whereas DHPs are selective for those found in the vasculature and therefore are vasodilators. How is diltiazem unique? Because it isn't selective for either VG calcium channels on cardiac myocytes or those on SM of the vasculature and so can exert both anti-arrythmic affects and vasodilation. How do Class 3 vasodilators treat angina? Mechanism of action: • systemic • act more on the arterial system than the venous system and so reduce total peripheral resistance • this therefore decreases cardiac work and so decreases the oxygen demand of cardiac myocytes • so there will be a significant enough reduction in cardiac work so that coronary circulation is able to maintain sufficient oxygenation of cardiac myocytes. • they also have a minor action - increasing coronary flow What is coronary flow? The movement of blood through the coronary circulation What are some unwanted side effects of VG calcium channel blocking vasodilators? • flushing of any part of the body due to vasodilation • effect of sm of GIT - reduction in motility Why don't calcium channel blockers used in the treatment of CVS conditions cause significant weakness in the skeletal muscle? Because these drugs target the particular isoform of L-type calcium channels found within the CVS and skeletal muscles express a different isoform. What does isoform mean in the context of cell receptors? An isoform in this context refers to a different molecular form or variation of a receptor protein that is dervied from the same gene. What are some other conditions that vasodilators are useful for? 1. 2. 3. 4. Raynaud's syndrome Erectile dysfunction (impotence) Male pattern baldness Improved cerebral function - treatment of haemorrhagic stroke and vascular dementia (only improve cerebral function if there is a preexisting reduction in cerebral function due to reduction in cerebral perfusion and so they are not useful as study drugs) How are vasodilators useful for the treatment of Raynaud's syndrome? Raynaud's syndrome is characterised by extreme vascular spasm within the extremities (most often in the distal parts of the fingers), usually in response to cold weather. This results in the fingers going white due to reduced blood flow and is often accompanied by extreme pain. Nifedipine, which is a DHP, is used to vasodilate and restore blood flow. Not always helpful - this suggests there are different mechanisms causing Raynaud's, i.e. different subtypes. Why are locally acting vasodilators useful in the treatment of erectile dysfunction? 1. To establish an erection the corpora cavernosa in the penis must fill with blood this is a specialised form of vasodilation 2. Under normal physiological circumstances this process is controlled by the release of NO and its subsequent action on cGMP 3. Interfering with this process allows us to affect the state of the smooth muscle of the vessels of the corpora cavernosa 4. Phosphodiesterase enzymes degrade cGMP found in smooth muscle cells 5. Therefore, inhibiting these leads to an increase in cGMP leading to vasodilation so more blood can flow allowing for an erection 6. It is important for this to be local as systemic vasodilation wouldn't counteract the problem What is a major example of a phosphodiesterase inhibitor? Sildenafil, otherwise known as Viagra: • PDE inhibitor that is selective for type 4 PDE (the type mainly found in genital tissue) • Therefore, localised action in male genital tissue • Vasodilates blood vessels of the corpora cavernosa facilitating an erection (when the individual is aroused - no arousal no erection) What is a severe side effect of sildenafil/viagra? The major drug interaction it has with nitrates, either pharmaceuticals like GNT or recreational drugs like poppers. This interaction can cause a fatal drop in blood pressure. What vasodilator is useful in the treatment of male pattern baldness? Minoxidil What is the mechanism of action for minoxidils treatment of male pattern baldness? Minoxidil is a vasodilator: • opens up potassium channels allowing potassium to leave cells • efflux of potassium from cells causes membrane hyperpolarisation • the calcium channels required to allow calcium to enter the cells of smooth muscle are voltage gated • therefore, membrane hyperpolarisation leads to the voltage gated calcium channels being less likely to be open • so less intracellular calcium and reduces contractility This treats hairloss because: - it is believed that the vasodilatory effect of minoxidil leading to improved scalp perfusion when applied topically could contribute to it causing hypertrichosis (increased hair growth) all though its action for this isn't fully understood. How does using vasodilators help with the management of a haemorrhagic stroke? • haemorrhage causes the blood vessels to go into vasospasm by contraction of smooth muscle • whilst this is helpful to reduce blood loss it lasts a long time • this presents a problem because all of the territory downstream of the blood vessels are not being supplied by blood and so the neurones there are under hypoxic conditions • at best this will cause them to dysfunctional and worst it will cause them to die • so it has been proposed it would be useful to have something to treat this and so research into using calcium channel blockers have been investigated for this How might using vasodilators be useful for the treatment of vascular dementia? Vasular dementia is due to changes in the vasculature of the brain and it has been suggested that maintaining cerebral blood flow by using vasodilators could hold off symptoms Which cells activate T cells? Dendritic cells What state do T cells enter the circulation after having matured in the thymus? As naive T cells (this means they have not encountered a pathogen). At this time the frequency of T cells for any given peptide/MHC complex is very low. When they leave they are either CD4, CD8, or T-Regulatory Cells. Why don't immature T cells produced in the bone marrow cause problems during their migration to the thymus? • Maturation Status: ◦ Immature T cells leaving the bone marrow are not fully mature and lack the ability to initiate immune responses. • Lack of Antigen Recognition: ◦ They have not undergone antigen recognition and selection processes, preventing them from recognizing specific antigens. • Controlled Migration: ◦ Migration to the thymus is tightly regulated, guided by homing receptors and specific molecules. • Immune Privilege of Thymus: ◦ The thymus is an immune-privileged site with mechanisms to create an environment conducive to T cell development without inducing immune responses. • Regulation of Immature T Cells: ◦ The immune system has checkpoints and control points to ensure proper maturation and selection before T cells become fully functional. • Microenvironment of the Thymus: ◦ The thymus provides specific signals for maturation, and its unique microenvironment minimizes the potential for inappropriate immune responses during T cell migration. What must naive T cells must do before they are useful? T hey must undergo: 1. Proliferation of antigen-specific cells 2. Differentiate to provide effector function What is needed to initiate proliferation and differentiation of naive T cells? Present antigens, via the MHC complex located on the surface of the antigen presenting cell the dendritic cell to the T cell What are the similarities between the different antigen presenting cells? • all very efficient at taking up antigens and processing them • they then are able to upregulate the level of MHC expression (on B cells it is already high), and also upregulate co-stimulation molecule activity. For the CVS, the efferent nerves come from... ... the ANS Given that reflex responses are superimposed on local influences, what are the local influences of the heart? • The local influences on the heart are its intrinsic beating (originates in SAN) and Starling’s Law. • These influence the HR and SV. Given that reflex reponses are superimposed on local influences, what are the local influences of the arterioles (resistance vessels)? The local influences on the arterioles are the substances released from the endothelial lining, metabolic influences (metabolites released from surrounding cells), the vascular smooth muscle response to myogenic stimuli. Given that reflex reponses are superimposed on local influences, what are the local influences of the capillaries? • The local influences on the capillaries are the forces across the capillaries e.g. osmotic and hydrostatic pressure. • These influence diffusion, filtration and exchange What are the local influences on veins (capacitance vessels)? Gravity, the respiratory pump, and the skeletal muscle pump When can the CNS initiate its own cardiovascular and respiratoru responses? Under certain conditions like emotional thoughts, previous experience, and volition or the willingness to engage in activities What are the two types of afferent pathways that affect the CVS? Cranial nerves and spinal nerves What are the two forms of efferent pathways that affect the CVS? 1. Parasympathetic vagal supply to the heart that affects the SAN and AVN 2. Sympathetic supply to the heart (SAN and AVN) and blood vessles What are the three hormones that are involved in CVS reflex responses? • Catecholamines - NAd and Ad • ADH - posterior pituitary • Renin-angiotensin system Sympathetic supply to the heart and blood vessels • SAN and AVN and blood vessels • Sympathetic preganglionic neurones originate in the spinal cord between T1L2 • They then synapse in sympathetic chain or in the prevertebral ganglia • Post ganglionic neurones then go to the heart, arterioles or venous vessels. • In order for the brain to influence sympathetic outflow, there are descending pathways in the spinal cord that influence sympathetic pre-ganglionic neurones. What would happen to the CVS in a spinal transection? • Lose the ability to reflex control the sympathetic nerve fibres that supply heart and BV • You would also lose tonic excitatory influence on sympathetic preganglionic neurones so your BP would be low. What level do simle reflexes in the CVS occur? The medulla What is tonic referring to in the nervous system? Continuos and baseline nervous impulses - either excitatory or inhibitory Nucleus Ambiguus in Cardiovascular Regulation Anki Card Summary: Topic: Nucleus Ambiguus in Cardiovascular Regulation Key Points: 1. Location: Nucleus ambiguus is located in the medulla oblongata, part of the brainstem. 2. Function: It is crucial for parasympathetic control of the heart. 3. Parasympathetic Output: Nucleus ambiguus provides parasympathetic efferent fibers to the heart via the vagus nerve (cranial nerve X). 4. Vagal Tone: Maintains vagal tone, influencing baseline heart rate during rest. 5. Baroreflex Control: Involved in baroreflex mechanisms, helping regulate blood pressure. 6. Coordination: Works in coordination with other cardiovascular centers in the brainstem. 7. Clinical Relevance: Imbalances in nucleus ambiguus activity may contribute to cardiovascular disorders. Rostral Ventrolateral Medulla (RVLM) in Cardiovascular Regulation 1. Location: RVLM is located in the rostral part of the medulla oblongata. 2. Function: It plays a crucial role in the sympathetic control of the cardiovascular system. 3. Sympathetic Output: RVLM contains sympathetic pre-ganglionic neurons that project to the sympathetic ganglia. 4. Blood Pressure Control: Influences blood pressure by regulating peripheral vascular resistance. 5. Baroreceptor Input: Receives input from baroreceptors, helping modulate sympathetic outflow in response to blood pressure changes. 6. Coordination: Works in conjunction with other brainstem centers, including the nucleus ambiguus, to maintain cardiovascular homeostasis. 7. Clinical Relevance: Dysregulation of RVLM activity may contribute to conditions such as hypertension. What level do the more complex reflexes of the cardiovascular system occur? At the level of the hypothalamus there are discrete integrating areas which are responsible for reflex patterns of response that we show under different circumstances: • Exercise pattern of response • Feeding/satiety pattern of response • Alerting/Defensive pattern of response • Thermoregulation pattern of response • Reproductive type behaviour These all have to communicate with nucleus ambiguus and the RVLM in order to affect vagal (parasympathetic) and sympathetic outflow to the heart and blood vessels. What does the baroreceptor reflex do? Homeostatically and autonomously regulates arterial blood pressure What are the two types of baroreceptors? Carotid baroreceptors and aortic baroreceptors The carotid baroreceptors • Located in the carotid sinus of the internal carotid artery, close to the bifurcation of the common carotid artery. • These receptors are supplied by the afferent sinus nerves, which then join the Glossopharyngeal nerve CNIX. Where are the carotid baroreceptors? The carotid sinus of the internal carotid artery, close to the bifurcation of the common carotid artery What nerve synapses with the carotid baroreceptors? Glossopharyngeal nerve (CN IX) The aortic baroreceptors? • Located in 2 patches in the aortic arch. • These receptors are supplied by the afferent aortic nerve fibres, which then runs in vagus nerve CNX. Where are the aortic baroreceptors located In two patches in the aortic arch What nerve recieves input from the aortic baroreceptors? Vagus nerve - CN X How do the baroreceptors work? They're stretch receptors: • They sense changes in the stretch of arterial walls caused by changes in pressure inside of artery. • When arterial blood pressure rises it leads to a stretch in the arteries, this cause an increase in afferent activity • The afferent activity carries information to the Nucleus Tractus Solitarius (NTS) (located in medulla) • This nucleus collects afferent information from lots of afferent nerve fibres and receptors that serve the CV, respiratory and GI system. How does the baroreceptor nerve impulses change according to arterial blood pressure? As arterial blood pressure increases, more impulses are sent. This is due to the fact that each baroreceptor is made up of multiple constituent neurones that each have a different threshold pressure before they start firing. Therefore, the higher the pressure, the greater the number of neurones reaching threshold, and so the greater the number of impulses. How do baroreceptors respond to the pulsatile nature of blood pressure? ABP is pulsatile due to systole and diastole - blood pressure is higher when the ventricles contract in systole and lower when they relax during diastole. Therefore, the baroreceptors increase their activity during systole and decrease during diastole - peaks and troughs in activity - but as long as ABP remains the same, the average number of impulses will remain the same. What is the brains autoregulatory range? 60-180 mmHg What is the autoregulatory range of the kidneys? 70-120 mmHg Why is the baroreceptor reflux important? Because it tonically keeps ABP down and continuosly buffers against changes in ABP that would otherwise be dangerously big Respiratory Pump in Cardiovascular Physiology Function: The respiratory pump aids blood flow within the thoracic cavity. 1. Inhalation: During inhalation, the diaphragm contracts, lowering intrathoracic pressure. 2. Venous Return: Lower intrathoracic pressure enhances venous return, aiding 3. 4. 5. 6. blood flow to the heart. Cardiac Filling: Increased venous return optimizes cardiac filling, crucial for maintaining preload. Exhalation: During exhalation, the diaphragm relaxes, raising intrathoracic pressure. Pulmonary Circulation: Increased intrathoracic pressure during exhalation assists blood flow in the pulmonary vessels. Cardiovascular Coordination: The respiratory pump complements the cardiac cycle, optimizing blood flow and cardiac function. What is sinus arrythmia? The interaction between inspiration and heart rate: • inspiration - heart rate goes up • expiration - HR goes down What are the two mechanisms of sinus arrythmia? 1. Cardiac vagal motor neurone activity 2. Involve the nucleus ambiguus Describe the central nervous mechanism of sinus arrythmia • There are central inspiratory neurones which are active during inspiration. • They are able to increase activity of inspiratory motor neurones (phrenic & intercostal nerves) that supply the inspiratory muscles (diaphragm and intercostal muscles) • Inspiratory neurones are also able send inhibitory influences to the Nucleus ambiguus (NA) • The NA is the nucleus that supplies vagus nerves to heart. • So, every time you breathe in there is an inhibitory influence to NA, which reduces vagal tone to heart ↑ HR. Describe how the reflex initiated by the pulmonary stretch receptors in respiratory airways leads to sinus arrythmia • The stretch receptors have vagal afferents. • The act of inspiration causes widening of the airways and therefore stimulation of the pulmonary stretch receptors. • The Pulmonary stretch receptors send impulses, via vagal afferents, to the Nucleus Tractus Solitarius (NTS). • From the NTS the impulses, that originated in pulmonary stretch receptors, are sent to NA and have an inhibitor effect on this nucleus. • Vagal tone is reduced and therefore heart rate increases At what arterial concentration of oxygen is the body considered to be under systemic hypoxia? 8.1kPa or 60mmHg What are the two groups of peripheral chemoreceptors? Coratid body and aortic body Where to the afferents from the carotid and aortic bodies run up to? The nucleus tractus sollitarius What are the peripheral chemoreceptors stimulated by? Fall in PaO2, increase in PaCO2, and a decrease in arterial blood pH Carotid body • Located at the bifurcation of the common carotid artery • Innervated by afferent fibres that joint the glossopharyngeal nerve (CNIX). • These afferents run up to NTS. Aortic body • Located in in the wall of the arch of aorta. • Innervated by afferent fibres that join the vagus nerve (CNX). • These afferents also run up to NTS. What do the peripheral chemoreceptors do when there is a fall in PaO2? Increase afferent stimulation to the nucleus tractus sollitarius Describe the reflex that occurs when respiration cannot increase but there is hypoxia • you get a primary cardiovascular reflex in response to peripheral chemoreceptor simulation. • The result of this reflex is bradycardia and vasoconstriction occurring via vagus nerve and sympathetic nerve activity to all major BV in body, except the brain (it has a weak sympathetic nerve supply). 1. 2. 3. 4. 5. 6. Mechanism: Peripheral chemoreceptors are stimulated, impulses sent to NTS. Pathways from Nucleus Tractus Sollitarius go to and stimulate the Nucleus ambiguus. This stimulates vagal activity which produces a reflex bradycardia (↓HR) Pathways from NTS also go and stimulate the Rostral Ventrolateral Medulla This produces an excitatory drive to sympathetic neurones that preferentially innervate the blood vessels (not heart) and causes vasoconstriction. Hence bradycardia and vasoconstriction. This is an oxygen conserving reflex: • Reducing O2 consumption of the heart by causing bradycardia • Vasoconstriction reduces the blood flow to various tissue reduces O2 Describe the action of the peripheral chemoreceptors when respiration can increase in response to hypoxia • In this scenario when peripheral chemoreceptors stimulate central inspiratory neurones which then increase inspiratory motor neurone activity respiration does increase. • Increase in respiration widens airways stimulates pulmonary stretch receptors. • This allows input from pulmonary stretch receptor afferent nerve fibres into NTS inhibit NA. • So now you have 2 inhibitory influences on NA and 1 excitatory influence. • The end result is HR goes up rather than down. • So in response to increase in respiration when chemoreceptors are stimulated there is an increase in HR but still there is vasoconstriction exerted via sympathetic fibres and RVLM. In what circumstances can systemic hypoxia occur despite the patient being able to increase respiration • If in Hypoxic atmosphere • If at High altitude • In you have a less severe respiratory disease – still have ability to increase respiration. What are 5 examples of when systemic hypoxia would occur due to respiration not able to match demand? • Patient given muscle relaxant when in surgery and so is paralysed - patient needs to be pump ventilated at a constant rate and depth • After high spinal transection - pt can no longer use phrenic nerves to control respiration and therefore cannot breathe unaided • Long dive under water • Fetus in utero if umbilical cord gets twisted • Patient with severe respiratory disease What are the local effects of hypoxia? • Decreased heart rate and contractility - local hypoxia to the SAN and myocardium • Cerebral vasodilation • Muscle vasodilation • Pulmonary vasoconstriction In respiratory disease a person who has hypoxia and is able to increase their respiration is known as... a pink puffer In respiratory disease a person with systemic hypoxia who is not able to increase their respiration is known as... a blue bloater Describe what happens when there is systemic hypoxia and respiration can be increased • The reflex effects of peripheral chemoreceptor stimulation on the heart are overcome by the effects of ↑ respiration on HR • Thus, you see an ↑ respiration + ↑ HR + generalised vasoconstriction (as a result of increase of sympathetic nerve activity): ◦ ↑ respiration helps to restore PaO2 (homeostatic part of reflex) ◦ ↑ HR and ↑ cardiac output that preferentially goes to brain (local vasodilation in response to hypoxia) • Because PaO2 better controlled when respiration can increase tissues do not become as hypoxic and pulmonary vasoconstriction less severe Hypoxic Pulmonary Vasoconstriction (HPV) • Definition: HPV is a physiological response to systemic hypoxia in the lungs. • Mechanism: ◦ Pulmonary arterioles in poorly ventilated areas sense low oxygen levels. ◦ Smooth muscle in arteriole walls contracts, causing vasoconstriction. • Purpose: ◦ Redirects blood flow to well-ventilated lung regions. ◦ Optimizes ventilation-perfusion (V/Q) matching for efficient gas exchange. • Outcome: ◦ Conserves oxygen by improving oxygenation of blood. • Clinical Relevance: ◦ Normal response but can contribute to pulmonary hypertension if excessive or prolonged. What is decreased respiration associated with? Bradycardia What are the three zones of trigeminal afferent innervation? Opthalamic, maxillary, and mandibular What zone of trigeminal afferent innervation is the most sensitive to the diving reflex? Maxillary How does the diving reflex work? • Trigeminal receptors are stimulated by Cold water on face/nose - evokes Diving Reflex • Stimulation of trigeminal afferents feeds into nucleus tractus sollitarius. This results in: ◦ Inhibition of central inspiratory neurones. This results in: ▪ Expiratory apnoea (breath out and stay in expired position). ▪ This also removes influences that increase HR (discussed previously). ▪ This cause by a pathway from nucelus tractus sollitarius to central inspiratory neurons. ◦ ↓ HR (↑ vagal activity) ▪ This is caused by a pathway from nucleus tractus sollitarius having an excitatory effect on cardiac vagal motor neurones. ◦ Vasoconstriction in tissues (GIT, Muscles, Skin, X Brain) due to ↑ sympathetic. ▪ This is caused by a pathway from nucelus tractus sollitarius having an excitatory effect to rostral ventrolateral medulla. • The sum total of this reflex is another O2 conserving reflex • If you are using this response e.g. when your face hits cold water that would be an advantage as you are conserving O2 at a time when you cannot breathe anymore O2 in. • Children have very strong diving reflex. ◦ Some children fallen under ice in Canada and rescued 30 mins later. This is due reduced O2 consumption. Expiratory apnoea • Definition: Expiratory apnea refers to the temporary cessation of breathing during expiration. • Characteristic: Breathing pauses after exhalation, leading to a brief period without airflow. • Physiology: ◦ Occurs during forced expiration. ◦ Results from decreased lung volume triggering reflexive apnea. • Clinical Relevance: ◦ Seen in conditions like asthma or chronic obstructive pulmonary disease (COPD). ◦ Can contribute to dynamic hyperinflation and respiratory distress. • Management: ◦ Address underlying respiratory conditions. ◦ Consider bronchodilators or other appropriate therapies. What are the two reflexes that cause bradycardia when there is decreased respiration? Diving reflex (trigeminal nerve stimulation by cold water) and a similar reflex triggered from receptors in the facial sinuses, larynx, and pharynx What occutions can trigger bradycardia upon decreased respiration other than the diving reflex? • sinus washing • irritant vapours • intubatin, bronchoscopy, laryngoscopy • when lumps of food get caught in pharynx or touch larynx • quadriplegic patients - occurs when mucous aspirated What is a way that supraventricular tachycardia can be stopped? Ice bag on the face - stimulates the diving trigeminal nerve reflex casuing apnea and bradycardia, which triggers heart rate to go back to normal. Alternatively: defib, carotid sinus massage, infusing adenosine What is steak house death? Some people are very sensitive to the two reflexes that cause bradycardia on decreased respiration and as such experience such a strong response they get complete expiratory apnoea and cardiac arrest resulting in death. Mouth to mouth ventilation can terminate apnoea and so reverse the reflex due to inspiratory input which restored breathing and heart rhythm. Steak house death is not due to food occluding airways but because of profound bradycardia and apnoea due to the bradycardia upon decreased respiration reflex being stimulated by food in touching the larynx or getting caught in the pharynx What are the two main effector arms of the baroreceptor reflex? • Autonomic nervous system - fast • Renin-aldosterone-angiotensin system: aldosterone has its action at the kidneys to alter blood volume (slow) and RAAS system acts on the vessels, e.g. angiotension 2 acts as a vasoconstrictor (fast) What factors need to be considered when beginning treatment for a newly diagnosed patient with hypertension? • Age of individual • Genetic heritage of individual • Comorbidities What are the drug classes for the treatment for hypertension? • Drug class A - RAAS system • Drug class C - calcium-channel blockers • Drug class D - diuretics What is the mechanism of action of ACE inhibitors? ACE inhibitors are Angiotensin Converting Enzyme Inhibitors so: • inhibit angiotensin converting enzyme (ACE) • ACE is needed to covert angiotensin 1 to angiotensin 2 • So decreased production of angiotensin 2 leads to a fall in both TPR and CO: 1. Angiotensin 2 is a vasoconstrictor so by reducing the concentration you get vasodilation and so decreased TPR - decreased ABP 2. Reduces aldosterone so less salt and water retension leading to a decreased venous return and therefore decreased cardiac output and therefore decreased ABP • additionally, they inhibit the degradation of vasodilator kinins such as bradykinin leading to vasodilation although this is minor What causes the dry cough side effect of ACE inhibitors? Dry cough: • Due to reduced degradation of Kinins. • This means Kinins are in the blood for longer periods and so enter the lungs. • The sensory nerve endings are irritated due to the presence of kinins which causes the dry cough • Some evidence suggests not all individuals are equally susceptible to developing drug cough – women> men, older>younger, east Asian> others. This may influence drug choice What is a significant drug interaction seen when using ACE inhibitors? Sudden severe hypotension on first dose of ACE inhibitor if individual is also taking a diuretic. This is very short lived so does not precluded combination being used. When are ACE inhibitors used? First line treatment for uncomplicated, mild hypertension in younger patients (under 55) What is the hydrostatic pressure at the arteriolar end of the capillary? 35 mmHg What is the hydrostatic pressure at the venular end of the capillary? 15mgHg What is the hydrostatic pressure of the interstitial fluid? Negligible - 0 mmHg What is the oncotic pressure of the capillaries? 25 mmHg What is the interstitial oncotic pressure? negligible - 0 mmHg Alterting/defence response extreme fight/flight/fright What does a typical alerting response consist of/ • Increase in respiration • An increase in CO, increase in BP due to decreased parasympathetic activity and increased sympathetic activity to the heart (reinforced by increased respiration stimulating vagal activity) • Reduction in skin temperature due to vasoconstriction • Increase in renal vascular resistance (vasoconstriction) (increased sympathetic activity) • GIT and splenic circulation reduced due to vasoconstriction (both due to increased sympathetic activity) • Reduction in limb muscle resistance (vasodilation) which is accompanied by an increase in muscle blood flow due to decreased sympathetic noradrenaline to the muscles and increased circulating adrenaline What is the alerting response often accompanied by? Facial expressions - apprehension, startled, suprised, acutely concerned, fear, aggression, defensive What can the alerting response be evoked by? Emotion, mental stress, visual, sound, pain, and novel environmental stimuli Is there always the same magnitude of response in the alerting response? No - it is graded with stimulus strength What is the alerting response accompanied by? Pupillary dilation, sweating, piloerection and, in extreme circumstances, urination and defaecation if a stimulus that previously caused an alerting response what can it show? • habituation - decrease reponse pattern with repeated exposure (type B) • sensitisation - response pattern gets bigger and bigger (type A) Can the alerting response be conditioned? Yes - typical pavlovian conditioning Why can the arterial blood pressure become very high during a strong alerting response? Because the baroreceptor reflex is suppressed What are the regions of the brain from which the whole alerting/defense response can be evoked by electrical stimulation via electrode activation? • the ventral hypothalamus • peri-aquaductal grey matter • the dorsal medulla What part of the thymus does V(D)J recombination occur? subcapsular cortex How is vasovagal syncope treated? • avoid fainting triggers • avoid hot crowded places • avoid alcohol • foot exercises, compression stockings, tensing leg muscles • adequate salt in diet • fludrocortisone acetate - expand blood volume • pacemaker - triggers when HR falls below predetermined level What is heart failure? Inability of the heart to supply adequate blood flow and therefore oxygen delivery to peripheral tissues and organs How common is heart failure in the UK? 900,000 What preportion of patients diagnosed with heart failure die within a year? 30-40% What are the symptoms of heart failure? • rapid weight gain • shortness of breath • increased swelling in the lower body • trouble sleeping • frequent dry, hacking cough • loss of appetite What is the most common caue of heart failure? Post MI development What happens in myocardial ischaemia? Tissue becomes: • hypoxic - low O2 • hypercapnic - high CO2 • glycolytic and acidotic - low pH • nutrient depleted (no substrate supply so reliant on glycogen and fat store metabolism) • tissue at risk of necrosis What are the causes of hypertension? Most common - post MI damage Others: Pressure overload - hypertension and aortic stenosis Contractile dysfunction - ischaemic heart disease, congenital cardiomyopathies What is the significance of a reverberating circuit found in the alerting/defence response? There is a reverberating circuit which goes from the amygdala, to the hypothalamus, and up round the stria terminalis, before going back to the amygdala. This helps us understand why and when a stimulus evokes an alerting response it doesn't just happen and then stop: • you tend to remain aroused for a period of time, and CV changes are prolonged for as long as you are showing the behaviour changes and experiencing the feeling of arousal • this is because the response reactivates itself through this reverberating circuit What is the amygdala? Part of the brain that is part of the limbic system and is concerned with emotional behaviour and how we deal with it What are the regions of the brain where the alerting/defence response can be evoked by exogenous electrical stimulation? • the ventral hypothalamus • peri-aquaductal grey matter • the dorsal medulla What regions of the brain can modulate the response patterns that are evoked in the main part of the defence area? Pre-frontal cortex and orbitofrontal cortex: • either inhibitory or excitatory • involved in sensitisation, habituation and conditioning Sensory input for the alerting/defense response goes to? Thalamus Dopamine output seen in the alerting/defense response comes from? Ventral tegmental area Top-down processing in the alerting/defense response happens where? • medial prefrontal cortex • orbital frontal cortex • anterior cingulate cortex Motor output seen in the alerting/defense response comes from? Peri aquaductal grey Central alerting ciruit in the alerting/defense response is? Amygdala and hypothalamus What is the clinical significance of the alerting/ defense response? It will: • increase arousal or apprehension in the individual • increased cardiac output which is preferentially redistributed to skeletal muscle allowing for a split second advantage before fight/flight before any exercise begun reinforces and increased muscle vasodilation Generally no benefit in everyday life: • increased ABP seen from this response in stress produces an acute risk for those with coronary artery disease (could cause MI or angina attack) and fragile cerebral blood vessels (stroke or burst aneurysm • chronically - repeated stress activitation of this response in individuals who do not habituate leads to the development of essential hypertension • however, those chronically stressed do show habituation of muscle vasodilation which then becomes vasoconstriction which contributes to the pressor response • large population studies show the pressor response to environmental and/or mental stressors increases the risk of developing chronic hypertension by 3-4 times over a 10-yr period What is the difference between the pressor response and the alerting/defense response? Describe chronic stresses impact on the pressor response Chronic stress can lead to a sustained activation of the alerting/defense response, which may not fully habituate in some cases. This lack of habituation can result in changes to the pressor response, particularly in muscle vasodilation. While other aspects of the alerting/defense response may not show habituation, muscle vasodilation does, leading to vasoconstriction and an overall increase in the pressor response. This increase is due to the increased total peripheral resistance, which elevates blood pressure. What type of response is seen from the rostral ventrolateral medulla during the alerting/ defense response? A very differentiated pattern of repsonse: • sympathetic activity to all tissues except muscle is increased • so there must have been an excitatory influence on those pre-motor neurones going to the gut and kidney and skin and an inhibitory effect of premotor neurones going to the skeletal muscle How does chronic stress lead to essential hypertension? Environmental stressors activate the defence areas in the brain (i.e. hypothalamus, amygdala), while multi-sensory cues are modulated by the higher centres. Genetic and other environmental influences module the response which will result in an increase in ABP. The increase in shear stress causees hypertrophy of vascular smooth muscle, narrowing the lumen of these vessels, increasing TPR. This causes a further increase in ABP, and the cycle continues, leading to chronic hypertension. How can essential hypertension be prevented in those experiencing chronic stress? Any activites that aid in relaxation and therefore reduce blood pressure such as exercise, relaxation therapy, music, yoga can help break the vicious cycle of chronic stress causing essential hypertension. What is essential hypertension? Essential hypertension, also known as primary hypertension, is the most common type of high blood pressure. It is characterised by abnormally high blood pressure that has no identifiable underlying cause and accounts for 85% of people with hypertension. What is secondary hypertension? Hypertension due to an underlying illness such as renal disease, sleep apnea, or thyroid problems - 15% of hypertension cases Vasovagal syncope Profound bradycardia and vasodilation leading to a massive drop in ABP that falls below the autoregulatory range of the brain tissue leading to the individual fainting (fainting aids in restoring blood flow to the brain). Causes: • sudden increase in vagal activity to the heart • a decrease in sympathetic activity to all blood vessels in all major vascular beds (causes vasodilation) Preceded by: • typically preceded by the alerting/defence response LBNP Lower body negative pressure HUT/LBNP test Head-up-tilt and lowe body negative pressure test. This test is used to assess the cause of a patients syncope, i.e. orthostatic hypotension, vasovagal syncope, or cardiac syncope. Procedure: 1. Patient is strapped to a tilt table 2. ECG attached to chest to monitor heart rhythm 3. Blood pressure cuffs attached to monitor blood pressure 4. Lower body negative pressure system attached to legs 5. Test has three phases 6. (1) Supine - pt lies on back for 20 mins while baseline vitals are recorded 7. (2) Upright - pt tilted to a 60 degree angle for 20 mins to simulate the upright position 8. (3) LBNP - pt reamins tilted upright while LBNP system gradually decreases the pressure experienced in their legs (-20mmHg, -40 mmHg, -60 mmHg for 10 mins each. Further challenges the patients bodies ability to maintain blood pressure in the upright position. If the patient has vasovagal syncope they would experience a faint at the point of the test where negative pressure is being applied as this causes greater venous pooling. This would cause a faint due to the baroreceptor reflex decreasing heart rate to conserve blood, central hypovolemia, and vasoconstriction all leading to reduced perfusion of the brain. Emotional fainting Vasovagal syncope can be evoked by strong emotion, e.g. fear or loss. Cortical influences facilitate the ventricular response, with an initial increase in heart rate and contractility followed by a vagal reflex, with a profound bradycardia and vasodilation. This occurs in extreme emotional stress, which may even cause cardiac arrest in profound fear/shock. Vasovagal reflex response 1. 2. 3. 4. 5. Stong defence response leads to increase in HR and contractility Reduced ventricular filling decreases EDV Torsion of ventricles stimulates high-threshold mechanoreceptors Increased vagal afferent activity to the medulla then to the lateral hypothalamus Reflex increase in vagal (PSNS) activity to the heart decreases HR, decrease in SNS activity to all blood vessels causes vasodilation 6. This evokes vasovagal syncope with a fall in ABP and cerebral blood flow, so the patient faints Adaptive processes in response to heart failure? Adaptive processes in response to heart failure, e.g. sympathetic stimulation, volume loading, and hypertrophy, can help the heart initially to mainting the CO, but can be deleterious in the long-term MI Myocardial infarction - happens when one or more of the coronary arteries suddenly becomes blocked, and a section of the heart muscle can't get enough oxygen Mechanism of MI Thrombus forms in one of the coronary vessels blocking a coronary vessel meaning an entire area of the myocardium usually supplied by the artery is at risk of necrosis. Coronary arteries are end arteries meaning that one part of the myocardium is only perfused by one artery. Ischaemia to an area of myocardium results in: • hypoxia • hypercapnia • glycolytic and acidotic (due to lactic acid build up) leading to a low pH • Nutrient depletion (no substrate supply, so reliant on glycogen and fat store metabolism - runs out) • Tissue at risk of necrosis - so speedy treatment vital How is a MI treated? Percutaneous Coronary Intervention: • catheter inserted into the arterial system (usually the femoral artery) and then threaded through the occluded coronary vessel • catheter punches through the thrombus physically breaking it up • once catheter has broken through a balloon is inflated so the coronary vessel is reopened • to ensure long term stability of this part of the vessel a stent is fitted • this allows for the reintroduction of blood flow to this part of the myocardium • the faster this procedure is done the better the outome for the patient (ideally done within 2 hrs of ischemic episode) • introduction of this procedure has meant that mortality due to acute MI has reduced but this means that more people are living with damaged hearts heart failure Describe the changes a thrombus causing MI would effect on an ECG We see changes to the S-T segment of an ECG: How can the return of oxygen facilitated by PCI treatment be damaging to the heart? • It can result in a massive burst in reaction oxygen species that can cause further cardiac myocyte death • if we protect those cardiac myocytes during the re-perfusion phase, we can then reduce infarct size down further to 5% area of the risk • this is what the research is focusing on now - providing this protection from re-perfusion injury What changes are seen to the starling ventricular function curve in heart failure? In a normal starling curve an increase in left ventricular end diastolic volume will lead to an increased stroke volume. However, in heart failure, an increase in LV EDV cannot lead to the same magnitude of SV increase for the same EDV, therefore the maximum SV is greately reduced. This is due to: • we have lost a proportion of cardiac myocytes • or they cannot contract as well In the early stages of heart failure, the SV and EF can sometimes by preserved, why? Due to a number of mechanism including the baroreceptor and blood volume loading (kidney and baroreceptor) How does baroreceptor reflex stimulation preserve SV and EF in early heart failure? Due to the partial loss of ventricular function leading to a decreased stroke volume and therefore cardiac output leading to a fall in arterial blood pressure. This causes baroreceptor unloading - the baroreceptor are not as activated so decrease the baroreceptor afferent activity input into the CNS. This triggers a number of reflexes: • reduction in vagal activity to SAN • increase in sympathetic activity to SAN - collectively these will increase heart rate and so increase cardiac output • Increase in sympathetic activity to ventricular cardiac myocytes (via B1 receptors) -> This leads to an increase in contractility increase SV Increase CO. Why is it that the autonomic action seen in the early stages of heart failure that maintains SV and EF and so CO eventually becomes deleterious? Because the autonomic action seen here leads to an increase in heart rate due to autonomic activity changes to the SAN. And autonomic (specifically sympathetic here) to the ventricular myocytes leading to increased contractility of cardiac myocytes - it leads to an increased cardiac workload and metabolic demand. This promotes pathological hypertrophy: • cardiac myocytes enlarge and lose shape • distance between capillaries and cardiac myocytes increases so they become more at risk due to reduced oxygen delivery However, recent studies suggest that beta-blocker treatment may attenuate cardiac hypertrophy Describe the hormonal action that combats heart failure? Fall in ABP -> decrease in baroreceptor afferent activity to the CNS -> reflex increase in symathetic activity to adrenal gland -> release of adrenaline (95%), NAd (5%) into the blood stream -> adrenaline acts on cardiac myocytes to increase contractility and also on SA nodal cells to increase heart rate -> pts with HF often have chronically high plasma catecholamine levels -> this increase in workload just to maintain SV and CO at rest promotes pathological hypertrophy. What are the consequences of persistent adrenergic stimulation of the heart that occurs during heart failure? Initially - increase in cardiac contractility and stroke volume Later - hyperphosphorylation of Ca2+ handling proteins can lead to dysfunctional calcium ion homeostasis, contractile dysfunction and arrhythmia Later - pathological hypertrophy Later - beta adrenoceptor internalisation -> loss of adrenergic sensitivity (this partially explains exercise intolerance in HF patients as they have no way to increase contractility during exercise). Describe the mechanism of delayed after depolarisation in heart failure leading to ventricular arrhythmia 1. Persistent sympathetic stimulation to cardiac myocytes causes an elevation in sarcoplasmic reticulum calcium lead. Therefore, sarcoplasmic reticulum calcium overload develops. This is when there is too much calcium stored in the sarcoplasmic reticulum. 2. This is dangerous because in normal physiology, calcium is only released during systole following the action potential and the opening of L-type calcium channels in the process of calcium induced calcium release. However, with pathologically high levels of SR calcium we can get spontaneous calcium release during diastole into the myocyte cytosol. 3. This calcium released spontaneously is extruded by the Na/Ca exchanger - this extrudes 1 calcium ion for three sodium ions leading to an increase in cellular net charge by one. This is a small depolarising current. 4. Therefore, a delayed after depolarisation starts - this is a small depolarisation during the diastolic interval 5. If the depolarisation is of significant magnitude, i.e. it causes the cellular charge to increase to threshold for voltage gated sodium channels, it triggers an AP this is called an ectopic action potential. 6. This pathological spontaneous activity that has developed can be transmitted to the rest of the myocardium 7. As HR worsens, more of these ectopic action potentials can generate in multiple myocytes across the ventricles leading to ventricular arrhythmia. How can the ventricular arrhythmia caused by ectopic action potential development be made worse? • When we have persistaent stimulation of beta-1 adrenoceptors we persistant action of PKA • This can result in hyper-phosphorylation of L-type calcium channels, RYR2 and PLB • RYR2 hyperphosphorylation results in RYR2 becoming very leaky so we are much more at risk of spontaneous caclium release during diastole which greately increases the risk of developing DADs and therefore ectopic activity. How is DADs and ectopic AP activity treated? Beta-blockers and calcium channel blockers should act to reduce the SR Ca2+ load, reducing the risk of DADs, ectopic activity and arrhythmia Combatting heart failure mechanism - blood volume loading - kidney and baroreceptor The decrease in SV, CO, and ABP in HF leads to: • baroreceptor unloading increasing sympathetic activity to the kidneys • reduction in wall tension in renal afferent arterioles • decrease in sodium delivery to macula densa in the kidneys Collectively these three mechanisms lead to an increase in renin release from granula cells in the juxtaglomerular apparatus resulting in an increase in the conversion of angiotensinogen (synthesised in the liver) into angiotension 1. Angiotension 1 is then converted by ACE into angiotension 2 -> pt with HR have chronically high plasma angiotension 2 -> increased angiotensin 2 goes to adrenal cortex increasing aldosterone secretion: • aldosterone acts in the pituitary to elevate ADH release • aldosterone and ADH will both act to increase water reabsorption Angiotensin 2 acts on hypothalamus to increase feeling of thirst and therefore pt will increase water uptake Blood volume increases -> increase in central venous pressure -> increase rate of cardiac filling -> increase in EDV -> increase in SV What is the consequence of blood volume loading in heart failure? Initial increase in blood volume leads to an increase in EDV -> acts to drive up SV to maintain SV at rest and preserve CO. However, as we keep increasing blood volume and so increasing EDV, we get to a point where we get beyond the plateau of the starling curve and we start to pull the myofilaments so far apart from each other we get a reduction in SV leading to SV not being maintained at rest. Therefore, we only want to increase our blood volume a little bit in HF. Oedema in heart failure • When there is a mismatch in left ventricular and right ventricular CO due to failing heart blood can start to accumulate in the systemic or pulmonary vascular system. • This leads to an increase in capillary hydrostatic pressure and an elevated capillary filtration leading to fluid accumulating in the interstitium -> oedema • this is worsened by the progressive increase in total blood volume seen in HF • oedema can be in the lungs and/or the periphery • accumilation of fluid in the lungs makes it harder to breath • as heart failure progresses this fluid not only accumulates in the interstitium but also alveoli and reduces the area for gas exchange Capillary filtration in the Pulmonary Circulation • Hydrostatic pressure in the capillary is truing to drive fluid out of the capillary • Hydrostatic pressure drops as we move from arterial to venous end • We also have oncotic pressure (colloidal osmotic pressure) which tries to pull fluid back into the capillary which remains constant along the length of the capillary • Under normal circumstances, in the pulmonary capillary, this is greater than capillary hydrostatic pressure • So there is a net movement of fluid from the interstitium back into the capillary • This is beneficial as we are constantly breathing in humidified air but because we are taking up fluid from the interstitium back into the capillaires we don't get a buildup of fluid in the lungs Pulmonary circulation in left sided HF • Left sided HF results in a mismatch between L CO and R CO (L CO < R CO) -> so we get an increase in blood volume in the pulmonary circulation • Hydrostatic pressure becomes raised in the pulmonary circulation and becomes higher than the oncotic pressure on the arterial side of the capillary bed • Leads to a net gain of fluid out of the capillaries and into the interstitium • Leads to pulmonary oedema developing - this is a major problem in HF What are the consequences of pulmonary oedema? • diffusion is impaired due to their being an increased distance for gas exchange -> if severe enough arterial hypoxia develops • arterial hypoxia results in shortness of breath - the feeling of oxygen starvation (primal emotion) • peripheral chemoreceptor activation on the fall of oxygen leads to increased ventilation, increased cardiac output, and decreased peripheral blood flow • hypoxia in the lungs leads to hypoxia pulmonary vasoconstriction and pulmonary hypertension leading to: increased workload of the heart (has to pump harder to overcome resistance), reduced blood flow to the lungs, damage to lung tissue (increased pressure damages alveoli), increased risk of blood clot How is pulmonary oedma treated? • 100% Oxygen administered to increase diffusion by increasing the concentration gradient • Longer term therapy includes loop diuretics, ACE inhibitors, and AT1R antagonists Mechanism of action of loop diuretics, ACE inibitors, and AT1R antagonists (ARBs) in the treatment of pulmonary oedema All work by modifying capillary hydrostatic pressure: 1. Loop diuretics - increase excretion of sodium and water leading to a decrease in blood volume and therefore CHP 2. ACE inhibitors - block the ACE enzyme, preventing the conversion of angiotension 1 to angiotensin 2, which reduces CHP 3. AT1R (ARBs) antagonists - block angiotensin 2 type 1 receptors, prevening vasoconstrictor effects of angiotensin 2, reducing CHP What are the consequences of cardiac hypertrophy? • Increases suceptibility to ischaemia (increase in the diffusion distance between cardiac myocytes and capillaries) • Increases incidence of arrythmias (decrease in ion channel density of cardiac myocytes) • increases incidence of sudden death How can the risk of cardiac hypertrophy developing be reduced? Reducing afterload with vasodilators, surgical valve replacement, and betablocker therapy (to reduce cardiac work). What happens to elastic arteries and the pulse with ageing? • Ateriosclerosis occurs with ageing leading to an increase in arterial stiffness and pulse pressure due to changes in vessel media (elastin becomes thin, broken, and/or disordered, and more collagen is laid down in its stead) • Mean ABP increases moderately with age due to increased TPR - systolic pressure increases to a greater extent than mABP due to increased stiffness and diastolic pressure begins to decline after 60 years Impaired baroreceptor sensitivity with ageing • Blunted baroreflex-mediated HR response to low BP means there is less increase in HR for a given fall in BP How does autonomic control of heart rate change with ageing? • There is age-related reduction in heart rate variability (HRV) in reesponse to breathing (RSA), cough, and valsalva • Reduction in baroreflex and vagal modulation of HR • Relatively greater loss of high frequency vagal parasympathetic component Ventricular diastolic function change with ageing • despite reduced early diastolic filling, stroke volume and cardiac output is usually preserved by atrial contraction in late diastole • consequences with disease - loss of atrial contraction with atrial fibrillation can reduce cardiac output by greater than 50%, tachycardia can threaten CO by shortening diastole so the ventricle is not adequately filled, risk of hypotension/syncope at onset of atrial fibrillation and other tachyarrhythmias Cerebral perfusion with ageing • Cerebral perfusion reduces with ageing, resting CBF lowers close to the threshold for ischaemia and therefore small changes in CBF can lead to symptoms • Cerebral autoregulation normally maintains CBF despite changes in BP reduced BP results in cerebral dilation, this is preserved with ageing although reset at higher levels with hypertension - BUT in some patients with hypotension paradoxical cerebral arteriolar vasoconstriction occurs with systemic hypotension The heart and ageing • Reduced pacemaker cells and altered sympathetic and parasympathetic activity leads to a decline in heart rate, reduced heart rate variability, and reduced maximum heart rate with exercise. • reduced ventricular compliance - hypertrophy of LV in response to arterial stiffness, drop out of myocytes, cross-linking of proteins • longer filling time and less tolerant of tachycardia and atrial fibrillation • less able to respond to changed in demand CVS - changes in sympathetic nervous system with age • increased secretion and decreased clearance of NA, therefore increased resting NA • Greater and prolonged NA response to upright posture BUT • Decreased responsiveness to sympathetic stimulation - no change in number of cardiac beta-receptors - reduced affinity for agonists at cardiac beta-receptors • impaired increase in heart rate localised at level of cardiac beta receptors Peripheral vascular resistance and ageing • blunted beta-adrenergic mediated skeletal muscle vasodilation • most older people do not have orthostatic hypotension despite reduced HR responses • BP on standing maintained by increased peripheral vascular resistance - but particularly vulnerable to vasodilator mediation Intravascular volume regulation with ageing Ageing predisposes to intravascular volume reduction - decline in plasma renin and aldosterone and increase in atrial naturetic peptide promotes renal sodium excretion; do not experience same sense of thirst as younger subjects - leads to dehydration and hypotension developing rapidly, e.g. during acute illness, fasting for procedures, and higher ambient temperatures. What changes are seen in the CVS with ageing? • heart changes • elastic arteries and pulse • baroreflex sensitivity • sympathetic nervous system • autonomic control of the heart rate • peripheral vascular resistance • ventricular diastolic function • intravascular volume regulation • cerebral perfusion What happens to large proximal elastic arteries and distal medium sized arteries with normal ageing? Large proximal elastic arteries like the thoracic and carotid arteries undergo outward hypertrophic remodelling: • lumen diameter increases • wall thickness increases • distensibility decreases Distal medium sized arteries like the brachial and radial artery undergo hypertrophic remodelling: • lumen diameter stays the same • wall thickness increases • distensibility stays the same Arterial wall distensibility The ability of an artery to stretch or expand in response to an increase in blood pressure. It is a measure of how much an artery can deform when it is stretched Artery wall elasticity The ability of an artery to recoil or contract after it has been stretched. it is a measue of how quickly an artery can return to its original size after it has been stretched Arterial wall compliance Broad term that includes both distensibility and elasticity. It is a measre of how easily an artery can be stretched and return to its orginal size. A compliant artery is one that is easily stretched and returns to its orginal size while an incompliant artery is one that is diffuclt to stretch and does not return to its orginal size Haematological changes in pregnancy 1. Plasma volume increases progressively preportional to the birthweight of the baby and the majority occurs prior to 34 weeks 2. Fall in haemoglobin, haematocrit, and erythrocyte count due to plasma volume increase being greater than erythrocyte increase 3. Despite haemodilation there is no change to MCV and MCHC 4. Platelet concentrations decrease but remain within normal limits for most women - a pregnant women is not considered to be thrombocytopenic until platelet count is less than 100x10 to the 9 cells/L 5. 2-3x increase in iron requirement 6. 10-20x increase in folate requirement 7. 2x B12 requirement 8. Balance in the coagulation system during pregnancy changes producing a hypercoagulable state in preparation for haemostasis following delivery. Cardiac changes in pregnancy 1. By 8 wks CO increased by 20% 2. Peripheral vasodilation occurs mediated by endothelium-dependent factors, including NO synthesis, upregulated by oestrodiol and vasodilatory prostaglandins 3. Peripheral vasodilation leads to a 25-30 fall in total peripheral resistance and to compromise a cardiac output increase of 40% occurs 4. Increased CO predominantly due to increased stroke volume 5. Max CO at 20-28 wks 6. Increased stroke volume due to early ventricular wall muscle mass increases and increased end diastolic volume 7. CO is maintained towards term despite decreased stroke volume and in late pregnancy due to increased heart rate 8. BP decreases in 1st and 2nd trimester but returns to prior levels by 3rd 9. Maternal position has a significant impact on the haemodynamic profile of mother and foetus 10. CVP remains the same 11. PVR decreases 12. Serum colloidal pressure (oncotic pressure) decreases by 10-15% 13. Susceptibility to pulmonary oedema increases 14. Pulmonary oedema is associated with increased preload and increased pulmonary capillary permeability 15. During labour there is a 15% increase in 1st stage and 50% in 2nd stage in CO 16. Post-delivery there is a 60-80% increase in CO due to relief of IVC obstruction and uterus contraction causing blood to empty into right atrium What normal physiological changes seen in pregnancy could be mistaken as being pathological by one not versed in CVS changes in pregnancy? • Bounding or collapsing pulse • Ejection systolic murmur • ECG changes: - Atrial and ventricular ectopics - Q wave smaller and inverted T wave in lead 3 - ST segment depression and T-wave inversion in the inferior and lateral leads - left-axis shift of QRS Why should pregnant women by nursed in the left or right lateral position? Because in the supine position, pressure of the gravid uterus on the IVC causes a reduction in venous return to the heart and a consequent fall in SV and CO. More haemodynamically stable in lateral position The precarious balance of the early immune system • By birth : ◦ The baby needs to have an immune system developed enough to fight infection ◦ The Mother can help – maternal antibodies can cross placenta • In utero : ◦ The mother’s immune system needs to be suppressed enough to prevent rejection of foetus. ◦ This is because the child is a mixture of their mother and father and so usually the mother’s immune system will recognise the baby as non-self.