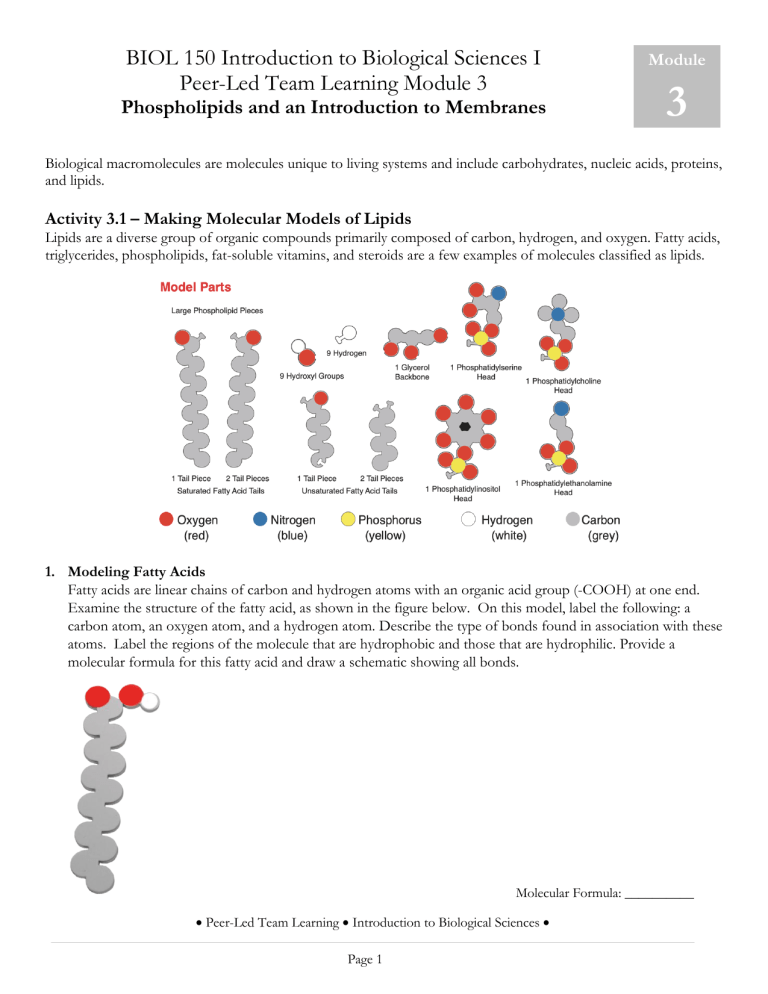
BIOL 150 Introduction to Biological Sciences I Peer-Led Team Learning Module 3 Phospholipids and an Introduction to Membranes Module 3 Biological macromolecules are molecules unique to living systems and include carbohydrates, nucleic acids, proteins, and lipids. Activity 3.1 – Making Molecular Models of Lipids Lipids are a diverse group of organic compounds primarily composed of carbon, hydrogen, and oxygen. Fatty acids, triglycerides, phospholipids, fat-soluble vitamins, and steroids are a few examples of molecules classified as lipids. 1. Modeling Fatty Acids Fatty acids are linear chains of carbon and hydrogen atoms with an organic acid group (-COOH) at one end. Examine the structure of the fatty acid, as shown in the figure below. On this model, label the following: a carbon atom, an oxygen atom, and a hydrogen atom. Describe the type of bonds found in association with these atoms. Label the regions of the molecule that are hydrophobic and those that are hydrophilic. Provide a molecular formula for this fatty acid and draw a schematic showing all bonds. Molecular Formula: __________ • Peer-Led Team Learning • Introduction to Biological Sciences • Page 1 2. Differentiating Fatty Acids and Carbohydrates Look at the chemical structures of steric acid, a common fatty acid, and the carbohydrate glucose. Compare the portion of carbon atoms to oxygen atoms in the table below. Steric Acid Substance Steric Acid Formula Glucose # of C atoms # of O atoms Ratio of C:O Ratio of C:H Glucose 2a. What do you notice about the amount of oxygen in a fatty acid compared to oxygen in a carbohydrate? 2b. What do you observe about the ratio of carbon to hydrogen in a fatty acid compared to a carbohydrate? 3. Modeling Triglycerides Triglycerides are neutral fats. Some triglycerides are considered fats and other oils. When a triglyceride is a solid at room temperature it is a fat. When a triglyceride is a liquid at room temperature it is an oil. The components that make up a triglyceride are glycerol and fatty acids. This activity will model the condensation (dehydration synthesis) reaction that results in the formation of a triglyceride. You will need to build a glycerol molecule, C3H8O3 and have three fatty acids ready. 1. 2. 3. 4. 5. 6. Begin with glycerol and three straight-chain fatty acids as reactants. A fatty acid is said to be saturated if the carbons comprising the tail are all singly bonded to each other. Remove one of the hydrogen atoms from the glycerol. Remove the hydroxyl group from one of the fatty acids. Combine the H and -OH. Join the fatty acid to glycerol. Repeat this process with the two remaining fatty acids. • Peer-Led Team Learning • Introduction to Biological Sciences • Page 2 3a. How many water molecules were formed in this reaction? 3b. What are the final products of the condensation (dehydration synthesis) reaction? 3c. Predict whether the resulting triglyceride would most likely be a fat or oil? Explain your reasoning. Substitute the third fatty acid tail with the two-part fatty acid tail. The post and hole connection in the two-part tail symbolizes a double bond between the carbons. When one or more double bonds are present between the carbons in a fatty acid, the molecule is unsaturated. Provide a molecular formula for this triglyceride and draw a schematic showing all bonds. Molecular Formula: __________ 4. Modeling Phospholipids Phospholipids are composed of two fatty acid tails connected to a glycerol backbone and a phosphate (polar) head group. This activity will model the condensation (dehydration synthesis) reaction that results in the formation of a phospholipid. You will need to build a glycerol molecule, C3H8O3 and have two fatty acids ready (one saturated and one unsaturated), and a phospholipid (polar) head group. 1. Begin with glycerol, the fatty acids, and phosphate (polar) head group as reactants. 2. Remove one of the hydrogen atoms from the glycerol. 3. Remove the hydroxyl group from one of the fatty acids. 4. Combine the H and -OH. 5. Join the fatty acid to glycerol. 6. Repeat these steps with the other fatty acid. 7. Remove the hydroxyl group from the phospholipid (polar) head and the final hydrogen atom from glycerol. Combine the H and -OH. 8. Bind the phospholipid (polar) head to the glycerol backbone. • Peer-Led Team Learning • Introduction to Biological Sciences • Page 3 Sketch the specific structural formula of the constructed phospholipid model. Activity 3.2 – Phospholipid Arrangement There are four major phospholipids, the comprise the plasma membrane. Phosphatidylcholine and sphingomyelin make up the outer leaflet of the membrane bilayer, while phosphatidylethanolamine and phosphatidylserine make up the inner leaflet. A fifth phospholipid, phosphatidylinositol is also found in the inner leaflet of the plasma membrane. Although phosphatidylinositol is a minor membrane component, it plays a major role in cell signaling. The general structure of a phospholipid is most often represented by phosphatidylcholine. 1. Phospholipid Monolayers One arrangement of phospholipids is to form a monolayer at an air-water interface. Using eight of the mini phospholipids and the beaker of water, show how the phospholipids would arrange themselves at the air-water interface. Sketch your result below. Air Water • Peer-Led Team Learning • Introduction to Biological Sciences • Page 4 2. Phospholipid Micelles Micelles form when phospholipids are mixed with water. Micelles can act as emulsifiers to form emulsions. An emulsion is a combination of two liquids that normally will not mix, such as soap and water, or oil and water. Using the same eight phospholipids, rearrange them in the beaker so that they are submerged in the water while still maintaining the correct hydrophobic and hydrophilic interactions. Sketch your result below. Water 3. Phospholipid Liposomes (Bilayers) Liposomes are a vesicle that is composed of a phospholipid bilayer, with water or another liquid in the center. Liposomes can be used to facilitate delivery of nutrients or pharmaceutical drugs. Construct a structure using phospholipids (you might need more than eight) that is both submerged and contains water on the inside. Water 3a. What factors influence the fluidity of a phospholipid bilayer? • Peer-Led Team Learning • Introduction to Biological Sciences • Page 5




