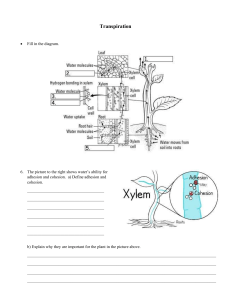
Soil Concepts Part one - The Inside Scoop By Dr. Scott Alan Barboza Abstract Introduction This is the first in a series of articles that will build our intuition about how soil and water interact in a pot. I’ve used familiar language throughout and avoided both math and aspects of soil chemistry. Although some of the jargon may not be familiar, the concepts are not hard and with a bit of familiarity, anyone can make informed soil choices. With the understanding, you’ll be able to answer questions like, “What grain size should I use?”, or “What does sieving do?”, or “Do I need a drainage layer?”, or “What does ‘fastdraining’ mean anyway?” for yourselves. If I can do it, so can you! In this article, we’ll learn some of the basic concepts of capillary action and what controls the amount of air and water in the pore space of your bonsai soil. These concepts will underpin some of the ideas we’ll discuss in later articles in the series. Soil questions come up all the time but few topics will raise the hackles of a mild-mannered bonsai artist more quickly. So right off the bat - be calm! I’m not here to tell you what you’re doing is wrong. Instead I’ll offer some information about how water and soil interact so we can all make informed choices. We’ll talk about what happens when we water and how the substrate properties and our pot choice can impact the amount of air and water available to the plant. We’ll talk about what aspects of the soil mix are healthy and what are not. Between “good” and “bad” there is a wide range of suboptimal but workable choices. I’ll try to make these distinctions where appropriate and known. I don’t care to recommend specific soils mainly because it’s your garden and you’re the one doing the watering. You’ll have to make your own choices in the end. But there are trade-offs between what’s optimal for us and what’s optimal for our plants. You can increase the air-filled porosity (more on this later) and get healthier growing conditions, but you’ll have to water more often and it’s easy to underestimate how much more your trees might need. AND the needs of the plant change over time depending on humidity, season, temperature, size and shape of the pot, and many other factors. The key is making informed choices and being aware of the trade-offs. That’s what we’ll try and accomplish. Figure 1: Right And the answer is ... That’s right - I’m not going to keep you in suspense. The message is that just a few factors control how much air and water are in our substrates: • Grain size, shape and composition • The depth of the container, the thickness of the drainage layer and the properties of the drainage layer material If we use a fine-grained substrate in a shallow container we’ll have waterlogged conditions in at least part of 24 50th Anniversary American Bonsai Society Figure 2: Left. Figure 3: Right. Figure 4: Below right. the pot for some time after watering. Not good, but we can mitigate it by adjusting some of the factors above. You’ll find that deep pots give you lots of options for optimal growing mediums, but those same substrates are suboptimal or even deadly in some of the shallow containers we use for bonsai. Why? What’s the solution? How fine-grained is too fine? What are the trade-offs? Let’s get started with the basics. It’s all about capillary action... but what the heck is capillary action anyhoo? Unless you’re a soil geek like me, you probably don’t think about this very much. Capillary action describes the behavior of fluids due to the forces of adhesion, cohesion and surface tension and it’s happening all around us. We need some intuition about this because it ties together everything else we’re going to talk about. We’ll just talk about the essentials here, but there are loads of places to go for more information (i.e., Lower, 2009; Khan, 2015). The good news is that it’s easier to understand than you might think. Many of us did the old capillary tube experiment in high school (Figure 1). Water is drawn up the tube against the force of gravity. The height it reaches is related to the diameter of the tube - it goes higher in narrower tubes. Why? Well, water molecules are polar meaning they have positive and negative sides (Figure 2). Since opposites attract, water molecules stick to each other and to other polar molecules. We call this “stickiness” cohesion (water molecules are attracted to water molecules) and adhesion (water molecules are attracted to solids - Perlman, 2016a ). See how the water curves up where it contacts the glass (Figure 1)? That’s due to adhesion between water and the glass tube. This adhesion causes an upward force creating a meniscus and drawing the water up the tube until the capillary forces are balanced by the force of gravity pulling the water down. Adhesion is stronger in narrower tubes so the water travels higher before it's stopped by gravity. Figure 3 depicts anthropomorphized water molecules climbing and pulling neighbors with them in a fun illustration of these concepts (MacBeth, 2012). Not only does water stick to glass, it sticks to your kids’ pants, soil, tree fibers and the fibers in a paper towel. Dip a towel into a glass of water and the water will be wicked in because of adhesion (Figure 4). In fact, it will continue to be absorbed until the pull of gravity balances the capillary 50th Anniversary American Bonsai Society 25 Figure 5: Below. Figure 6: Right. forces, just like in the capillary tube experiment. All sorts of processes depend on cohesion and adhesion, including the movement of water to the tops of trees and the drainage of tears from ducts in the corners of your eyes (Perlman, 2016b). Spill a bottle of Gatorade and capillary action is there to help you out. First, surface tension collects it in a nice puddle on the table instead of a thin film spread out over the floor. What’s surface tension? Well, it’s just a way to describe the cohesive forces between molecules at the interface between two immiscible fluids. Think about the water molecules at the surface of a drop of water. Since they have air molecules on one side and water molecules on the other, they cohere more strongly to the water. That “extra” cohesion is called surface tension and it’s why immiscible fluids form spherical shapes - like a rain drop or a bubble or how water domes up before it spills over the edge of a glass. Second, when you grab a towel, it’s adhesion that wicks the stuff off the floor. Thanks, capillary action! For some materials (Figure 5) adhesion is much stronger than cohesion. Water will spread out like a film on these “high wettability” materials. For other surfaces (Figure 5), cohesion is much stronger than adhesion. Water on the surface of these “low wettability” materials will bead up like a ball. Think about a freshly waxed car surface. Before you wax, water spreads out in a film because the painted surface has a high wettability. After you wax you have a low wet- 26 50th Anniversary American Bonsai Society tability surface, so the water beads up and runs off. Lot’s of plants have low wettability leaves that achieve the same effect (i.e., Figure 6). Ever notice how soils like dry peat moss tend to shed water rather than allowing water to penetrate the soil (Figure 7)? This is because peat moss has a low wettability when it’s dry. Once the water enters the micropores it’s wetting character changes making it much easier to water. Sometimes people call these “hydrophobic” soils. Well, what does this have to do with watering my bonsai? Capillary action in soil works the same as in capillary tubes. Because of adhesion, water is wicked into spaces between the grains the same way water is wicked into the holes in a sponge. The height the water rises is dependent on the pore size. The smaller the pores, the higher the capillary rise. But here’s the difference - soil has a whole range of pore sizes (Figure 8). It’s far more complicated than in a constant diameter capillary tube, it’s like ALL the capillary tubes are there at the same time! So at any point in the soil, some of the pores have water in them and some don’t. If most of the pore spaces are filled with water, the water saturation is said to be high. If most are filled with air the saturation is low. You might ask, “But I don’t wick water into my bonsai. I water from the top like a normal person. So does any of this matter?” I’d answer, “It amounts to the same thing.” We’ll get into why that is in the next article in the series. Conclusions In summary, there are two key lessons for the month: 1. If your soil has mostly small pores, the water saturation everywhere will be higher. If it has mostly large pores the water saturation will be lower. Note that the number of pore spaces doesn’t matter - just their size. Your soil can have a high porosity, but if the pore size is small the water saturation will be high too. 2. Since capillary action is balanced by gravity, the saturation at the base of the soil column will always be higher than at the top. If we remember these two ideas for the next article we’ll be in good shape. That wraps it up for this installment. We’ve covered some basic elements of capillary action and discussed how pore size and gravity control the distribution of air and water in the soil. But what controls pore size? Why do people grow their trees in those rocks? What happens when we water our plants? Don’t we just need a “fast draining” soil? What does a “drainage layer” do - does the soil drain faster? Can’t we just put more holes in the bottom of the pot? And what’s all the fuss - how important really is any of this stuff to my trees? All these topics and more will be discussed in upcoming articles in this series. Figure 7: Above left. Figure 8: Above right. References Apel, M. 2006. "Water Beading on a Leaf "[ONLINE]. Available at: https://upload.wikimedia.org/wikipedia/commons/8/8c/Dew_2.jpg [Accessed 19 December 2016]. Khan, S. 2015. "Capillary Action and Why We See a Meniscus." The Khan Academy [ONLINE]. Available at https://www.khanacademy.org/science/ biology/water-acids-and-bases/cohesion-and-adhesion/v/capillary-actionand-why-we-see-a-meniscus [Accessed 19 December 2016]. Lower, S. 2009. "Liquids and their interfaces." Chem1 Virtual Textbook. [ONLINE] Available at: http://www.chem1.com/acad/webtext/states/liquids. html#SEC3. [Accessed 19 December 2016]. MacBeath, M. 2012. "Anthropomorphized Water Molecules." [ONLINE]. Available at: http://rieson.blogspot.com/2012/12/capillary-action.html [Accessed 19 December 2016]. Perlman, H. 2016a. "Adhesion and Cohesion of Water.", The USGS Water Science School, U.S. Department of the Interior/U.S. Geological Survey [ONLINE]. Available at: http://water.usgs.gov/edu/adhesion.html. [Accessed 19 December 2016]. Perlman, H. 2016b. "Capillary Action.", The USGS Water Science School, U.S. Department of the Interior/U.S. Geological Survey [ONLINE]. Available at: http://water.usgs.gov/edu/capillaryaction.html. [Accessed 19 December 2016]. Stiehler, B.J. 2013. "Wetting Agent." [ONLINE]. Available at http://http:// highlandsccgolfmaintenance.blogspot.com/2013/05/wetting-agent.html [Accessed 4 January 2016]. “The Chemistry of Life.” 2013. Retrieved from https://dlc.dcccd.edu/biology1-2/water. [Accessed 4 January 2017]. 50th Anniversary American Bonsai Society 27