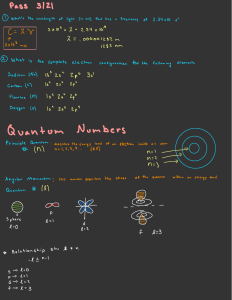
www.apchemsolutions.com Lecture 2 Atomic Theory II Tutorial 1) A photon travels with a frequency of 8.57 x 1014 Hz. a. Find the wavelength of this photon in nm? c = λν c λ = ν 3 × 1 0 8 m /s 8.5 7 × 10 1 4 s − 1 λ = 3 .5 0 × 1 0 − 7 m λ = λ = 3 .5 0 × 1 0 2 n m b. Find the energy contained by a single photon. Put your answer in kJ. E = hν E = (6.63x10−34 Js) (8.57 x 1014 s −1 ) E = 5.68 x 10−19 J E = 5.68 x 10−16 kJ 2) An electron drops from n = 5 to n = 3 in a hydrogen atom. a. Find the energy contained by the photon that is released during this transition. −2.178 ×10−18 J 52 E5 = −8.712 × 10−20 J E5 = −2.178 × 10−18 J 32 E3 = −2.420 ×10−19 J E3 = ΔE = E3 − E5 ΔE = − 2.420 × 10−19 J − (−8.712 ×10−20 J) ΔE = − 1.549 × 10−19 J b. Find the frequency of the electromagnetic radiation that is released. E = hν ν= E 1.549 ×10−19 J = = 2.34 × 1014 s −1 h 6.63 ×10−34 Js c. At what speed does this electromagnetic radiation travel? (3x108 m/s) The speed of electromagnetic waves (light) is a constant. © 2009, 2008 AP Chem Solutions. All rights reserved. 1 www.apchemsolutions.com d. What is the wavelength of this electromagnetic radiation? c = λν c λ = ν 3 .0 0 × 1 0 8 m /s λ = 2 .3 4 × 1 0 1 4 s − 1 λ = 1 .2 8 × 1 0 − 6 m λ = 1 .2 8 × 1 0 3 n m 3) An electron is traveling with a velocity of 3.92 x 106 m/s. (mass e- = 9.11 x 10-31 kg) a. What is its wavelength in nm? λ= h 6.63 ×10−34 Js = = 1.86 ×10−7 m = 1.86 ×102 nm mv (9.11×10−34 kg)(3.92 × 106 m/s) b. What is its frequency? c = λν c ν = λ 3 × 1 0 8 m /s 1 .8 6 × 1 0 -7 m ν = 1 .6 1 × 1 0 1 5 s − 1 ν = 4) Find the momentum of an electron with a wavelength of 232 nm. ρ = mv λ= h h = mv ρ ρ= h λ = 6.63i10−34 Js = 2.86 ×10−27 kg ⋅ m/s −9 232i10 m 5) How many possible orientations exist in a p-sublevel? The angular momentum quantum number (l) for a p-sublevel is 1. The magnetic quantum numbers range from - l to + l . For a p-sublevel the magnetic quantum numbers are -1, 0, and +1. Thus, there are THREE possible orientations in the p-sublevel. © 2009, 2008 AP Chem Solutions. All rights reserved. 2 www.apchemsolutions.com 6) What is the lowest principal quantum number that can have p-orbitals? n-1 = l n = l +1 n=1+1 n=2 7) If n=5: a. What are the possible angular momentum numbers? The angular momentum numbers range from 0 to n-1. If n = 5 the possible angular momentum numbers are: 0, 1, 2, 3, and 4. b. What do these numbers represent? The angular momentum numbers represent the shape or type of orbital. 0 is an s-orbital, 1 is a p-orbital, 2 is a d-orbital, and 3 is an f-orbital. 8) If l = 3: a. What are the possible magnetic quantum numbers? -3, -2, -1, 0, 1, 2, and 3 b. What do these numbers represent? These numbers define the orientation of the orbital in three-dimensional space. In this case, there are seven different orientations (an f-sublevel). 9) What are the possible angular momentum, magnetic, and electron spin quantum numbers associated with a single electron in the s-orbital of n = 2. l = 0 (An s-orbital is defined by l = 0) m = - l to + l , so m = 0 (there is only one orientation for an s-orbital) ms= + ½ or – ½ (the one electron could be spin up or spin down.) 10) What are the possible angular momentum, magnetic, and electron spin quantum numbers associated with a single electron in an f-orbital of n = 5. l = 3 (l = 3 defines an f-orbital) m = - l to + l , so m = -3, -2, -1, 0, +1, +2, or +3 (there are seven possible orientations for f-orbitals) ms= + ½ or – ½ (the one electron could be spin up or spin down.) © 2009, 2008 AP Chem Solutions. All rights reserved. 3