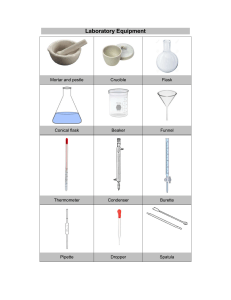
College of Engineering- Department of Civil Engineering GROUP 5 Members: Amponin, Jhon Rey De Guia, Shereelou Delos Santos, Justine Macalalad, Angelene D. Ricero, Suzanie A. PRE LAB ACIDITY I. INTRODUCTION Acidity is a chemical property that characterizes the concentration of hydrogen ions in a solution. Acidity in water in essence arises from the presence of dissolved acidic compounds. These compounds can lead to the decrease in the water’s pH which indicates its acidic nature. II. MATERIALS: Apparatus ● ● ● ● ● ● ● ● ● Analytical Balance Burette with stand Beaker (10 ml) Dropper (provided) Volumetric Flask Conical Flask Funnel Magnetic Stirrer (if available) Magnetic Bar (if available) Chemical and Reagents ● ● ● ● III. NaOH Solution (available in lab) Methyl Orange Indicator (provided) Distilled Water (not provided) Water Sample (needed: 10ml) COSTING College of Engineering- Department of Civil Engineering QUANTITY MATERIAL COST 1 METHYL ORANGE INDICATOR 216 6 DROPPER ₱8.00 x 6 (48) CONTRIBUTION: TOTAL COST 30 = 264 30 Total Contribution=8.8 or 9 pesos (each) IV. PROCEDURES: 1. Preparation: 1.1. Set up a clean and dry workspace. 1.2. Ensure that the burette, conical flask, and other glassware are clean and free from any contaminants. 2. Prepare the 1N NaOH solution: 2.1. Get the analytical balance and put the petri dish on it. 2.2. Weigh 4 grams of sodium hydroxide using the analytical balance. 2.3. Add the weighted NaOH to the 1-liter volumetric flask. 2.4. Pour 400 mL of distilled water into the flask. It is only an initial volume to ensure that the solution dissolves completely. 2.5. After the NaOH has dissolved completely, add more distilled water to the flask until the volumetric flask reaches the 1-liter mark. College of Engineering- Department of Civil Engineering 3. Measuring the Water Sample: 3.1. Using a graduated cylinder or a measuring pipette, carefully measure 10 ml of the water sample and transfer it to a clean and dry beaker. 4. Adding Methyl Orange Indicator: 4.1. Using a dropper, add 2 to 3 drops of 1% Methyl Orange indicator solution to the water sample in the beaker. Swirl gently to mix. 5. Transfer to Conical Flask: 5.1. Carefully the solution from the beaker into a clean conical flask. 6. Preparing the Burette: 6.1. Place the burette in a burette stand. 6.2. Using a funnel, fill the burette with the 0.1 normal NaOH solution until the zero point. Ensure that the burette is completely filled, and there are no air bubbles in the tip. 7. Titration Process: (A) Setting Up Magnetic Stirring: 7.1. Place a magnetic stirrer below the burette. 7.2. Carefully place a magnetic bar into the conical flask containing the water sample and indicator. 7.3. Power on the magnetic stirrer to ensure uniform mixing of the solution. 7.4. Open the stopcock of the burette to allow a controlled flow of the NaOH solution. 7.5. Continue adding the NaOH solution until the color of the solution turns pink. The pink color indicates that the water has reached neutral pH. (B) Manual Swirling: 7.1. In case of the absence of the magnetic stirrer at the laboratory, simultaneously swirl the flask manually with your hand. 7.2. Open the stopcock of the burette slightly to allow a slow and controlled flow of the NaOH solution. 7.3. Add the NaOH solution dropwise into the conical flask containing the water sample. Swirl the flask gently after each drop. 7.4. Continue adding the NaOH solution until the color of the solution in the conical flask changes from its initial color to pink. The pink color indicates that the water has reached neutral pH. 8. Stopping the Titration: 8.1. As soon as the pink color appears and persists for about 30 seconds, immediately close the stopcock of the burette to stop the flow of NaOH solution. 9. Recording the Reading: College of Engineering- Department of Civil Engineering 9.1. Carefully read and record the initial and final burette readings. The difference between these readings represents the volume of NaOH solution required to neutralize the acidity in the water sample. 9.2. You can use this as a guide for the readings; Volume of water Sample(V): 10 ml Consumed titrant (a): (?) ml Normality of NaO(N): 0.1 N V. FORMULA TO USE: Calculating Acidity: Acidity = VI. 𝑁•𝑎 𝑉 • 1, 000 𝑚 𝑚𝑜𝑙/𝑙 GUIDE QUESTIONS: 1. What observations did you make? 2. What are the differences you noticed between the water samples and how they reacted to the different solutions? 3. How can sources of error in the titration process be identified and addressed to improve the accuracy and precision of the results? 4. How can the titration data be interpreted to draw conclusions about the properties of the substances involved in the experiment?

![BAI HAYYAT PAGLALA - [Template] [Template] Chem 1101B Exp. 4.](http://s1.studylib.net/store/data/025612661_1-90f80f72c7afd19687516e5c2a09b92e-300x300.png)