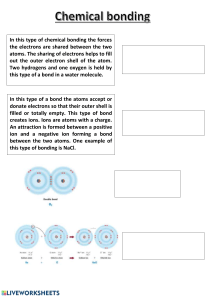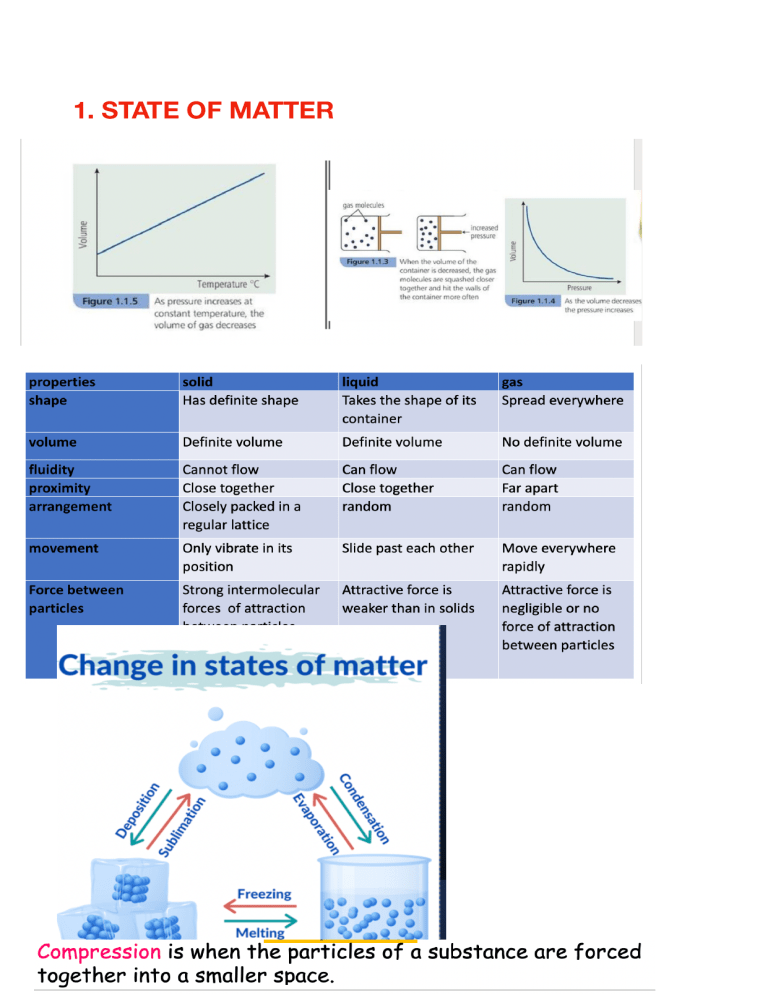
1. STATE OF MATTER Page 1 What is Brownian Motion? All matter is made of tiny particles. The particles are atoms, ions, or molecules. In a liquid or gas, the particles move at random. The random motion of particles is called Brownian Motion. It can be seen by looking at smoke particles in the air through a microscope. •The gas molecules move at high speed colliding with other molecules. •In doing so they continually change their velocity (speed and direction), giving rise to the observed Brownian motion or random walk behavior. 2. Atoms, elements and compounds Page 2 proton number/ atomic number is the number of protons in the nucleus of an atom of the same element mass number/nucleon number is the total number of protons and neutrons in the nucleus of an atom of the same element What is an isotope? Atoms of the same element with the same number of electrons and protons but a di erent number of neutrons Chemical properties (how they react) are mostly determined by the number of electrons, hence chemical properties are identical Physical properties are mostly determined by the atomic mass, so these change Why do isotopes have the same chemical properties? The same number of electrons Why do isotopes have di erent physical properties? Di erent number of neutrons, di erent atomic mass Atoms with the same number of electrons in their outermost shell belong to the same group in the periodic table The period number determines how many shells there are The number of electrons in the outermost shell determines the way the element will react (chemical properties) Noble gases have a full outer shell (stable) and, therefore are very unreactive The electrons orbit around the nucleus of the atom in di erent Energy Levels or Shells The shells ll up in order, the lowest energy level (innermost shell) lls up rst 1st Shell – Maximum 2 Electrons 2nd Shell – Maximum 8 Electrons 3rd Shell – Maximum 8 Electrons 4th Shell – Maximum 18 Electrons •Atoms with an incomplete outer shell are unstable •They can easily react with other atoms by losing, gaining, or sharing electrons •When they lose or gain electrons they form IONS •Ions are atoms that have lost or gained electrons and have a charge as well as a full outer shell •Atoms in the same group in the periodic table have the same number of electrons in their outer shell; they want to lose or gain the same number of electrons to become stable The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. ionic lattice: positive and negative ions [1] regular pattern / opposite charges closer than the same charge [1] ff fi fi ff ff ff fi ff Page 3 The positive and negative ions formed by the complete transfer of electrons are strongly attracted to each other. This electrostatic attraction is called ionic bonding. Lattice: /repeated/ pattern/ framework / periodic / ordered/ alternating/organized; (of) particles/ atoms / molecules/ions / cations/anions; v Page 4 Page 5 Page 6 Page 7 Silicon dioxide has a similar structure as diamond tetrahedral. Metallic bonding marking scheme: positive ions or cations NOT atoms or cores or nuclei layers or lattice or regular pattern delocalised or free or mobile electrons or sea OR positive ions or cations NOT atoms or cores or nuclei attraction between ions and electrons delocalized or free or mobile electrons or sea the attraction/electrostatic bonding must be between ions and delocalized electrons, between cations and anions does not score ACCEPT bond if quali ed - electrostatic bond, etc. if molecular or molecules then cannot score cation mark 3. Stoichiometry The relative atomic mass (Ar) of an element is the average mass of the isotopes of an element compared to 1/12th of the mass of an atom of Carbon -12 The relative molecular mass(Mr) of a substance is the sum of the relative atomic masses of the elements present in the molecule Relative formula mass (Mr) in the case of ionic compounds fi Page 8 Page 9 Page 10 In a chemical reaction, one of the reactants is often added in excess and is not completely used up in the reaction. The amount of product formed is determined by the amount of reactant that is not in excess and so is used up completely in the reaction. This is called the limiting reactant. Explain which of the reactants is in excess. Use the balanced equation: CaCO3 + 2HNO3 → Ca(NO3)2 + H2O + CO2 There are 2 mol of HNO3 for 1 mol of CaCO3 0.063 mol of CaCO3 would need: 2 ´ 0.063 = 0.126 mol HNO3 to react and only 0.1 mol is present. So CaCO3 is in excess and HNO3 is the limiting reactant. Page 11 Page 12 Page 13 4. Electrochemistry Page 14 Page 15 Page 16 Page 17 5.Chemical energetics: Exothermic: Endothermic: Page 18 Page 19 6.Rate of reaction Page 20 Page 21 7. Acids and bases Page 22 Page 23 Page 24 Page 25 8. Periodic table Page 26 Page 27 Page 28 9.Organic chemistry: Page 29 Page 30 Page 31 Page 32 Page 33 Page 34 10. Expermintal technique and chemical analysis Page 35 Page 36 Page 37 Page 38 Page 39 Page 40