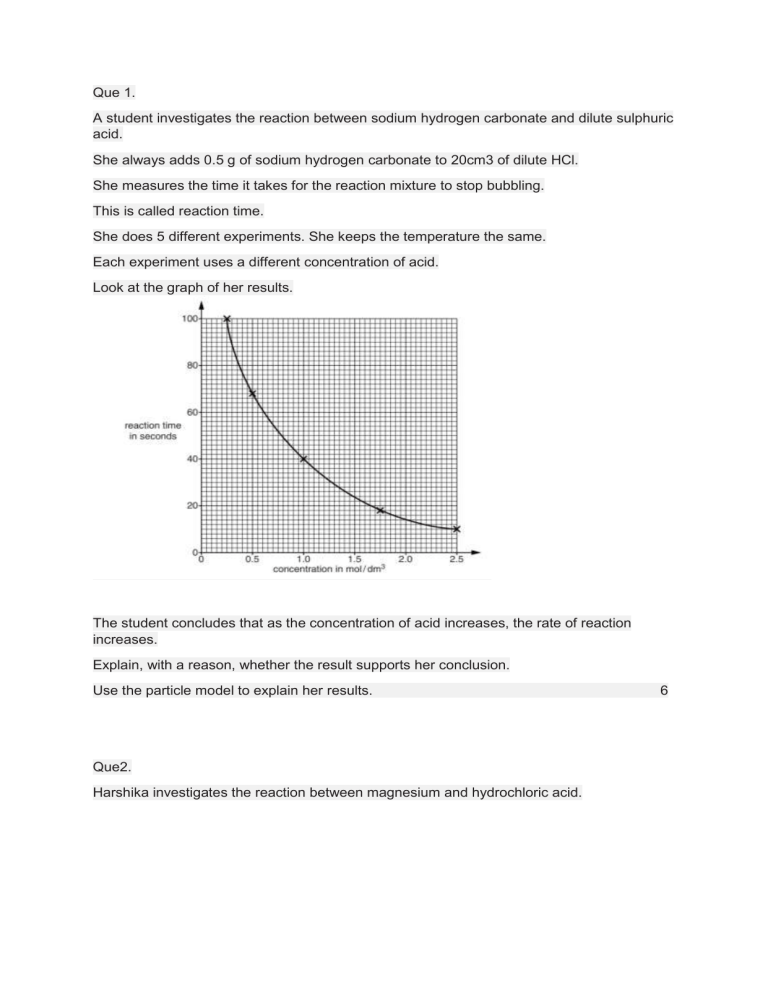
Que 1. A student investigates the reaction between sodium hydrogen carbonate and dilute sulphuric acid. She always adds 0.5 g of sodium hydrogen carbonate to 20cm3 of dilute HCl. She measures the time it takes for the reaction mixture to stop bubbling. This is called reaction time. She does 5 different experiments. She keeps the temperature the same. Each experiment uses a different concentration of acid. Look at the graph of her results. The student concludes that as the concentration of acid increases, the rate of reaction increases. Explain, with a reason, whether the result supports her conclusion. Use the particle model to explain her results. Que2. Harshika investigates the reaction between magnesium and hydrochloric acid. 6 She adds a piece of magnesium ribbon to HCl in a beaker. Harshika measures the time it takes for all the magnesium ribbon to react. This is the reaction time. She does five different experiments. Look at Harshika’s prediction. Look at Harshika’s results: a. Explain if Harshika’s result support her prediction. Use the reacting particle modelto explain the difference between the reaction time in experiment 3 and 4. 6 b. Harshika repeats her experiment 1. This time she uses acid at a higher temperature. Explain, using the particle model, what happens to the rate of a reaction. 2. Que 3. Nitrogen molecules react with oxygen molecules. Nitrogen monoxide molecules are made. N2 +O2 -------------2NO This reaction is endothermic. a. Explain in terms of bond making and bond breaking, why this reaction is endothermic? 3 b. Nitrogen and oxygen molecules react extremely slowly, even at 200 degree Celsius. The reaction between N and O becomes faster as both the temperature and the pressure increase. Explain why, using the reaction particle model. 6 Que 4. In which state of matter are the particles furthest apart? 1 Que 5 Which state of matter has a fixed volume but no fixed shape? 1 Que 6 Which of these changes could be brought about by heating a substance? a. Condensing b. Freezing c. Melting 1 Que 7 Which of the following changes involves the transfer of energy to particles from the surroundings? a. Freezing b. Melting c. Condensing Que 8. Which of the following is an example of a chemical change?