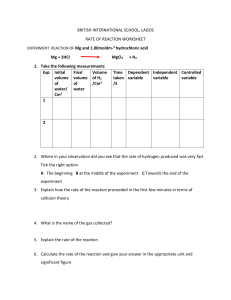
IBSL/HL Chem Invest 6.1A: Rate Dependant Factors INTRODUCTION: Thiosulfate ions react in acid solution to produce a precipitate of sulfur according to the equation: S2O32–(aq) + 2 H+(aq) → H2O(l) + SO2(g) + S(s) The rate of the reaction can be followed by measuring the time taken for a fixed amount of sulfur to be produced. This practical first examines the effect on the rate of the reaction when the concentration of thiosulfate ions is altered at a fixed temperature. Then it examines how the rate varies with temperature for a fixed concentration of thiosulfate ions. ENVIRONMENTAL CARE: Sodium thiosulfate is known as photographers 'hypo' for fixing developed films and prints. To minimize pollution the concentrations of thiosulfate ions have been kept low and only very small amounts of sulfur dioxide are evolved. The residues can be disposed of down the sink. SAFETY: There are no particular hazards associated with this practical except that you should avoid breathing in any sulfur dioxide that is evolved while you are waiting for the cross to disappear. PROCEDURE: 1. Concentration. a. Measure 50 cm3 of 0.20 mol dm-3 sodium thiosulfate solution into a conical flask. b. Place the flask onto a white piece of paper or note card marked with a black “X”. c. Measure out 5 cm3 of 2.0 mol dm-3 hydrochloric acid into a measuring cylinder and then add the acid to the thiosulfate starting a stop-clock at the same time. d. Swirl the contents of the flask initially to mix and then allow the flask to remain still on the tile. e. Look down vertically through the solution and time how long it takes for the cross to “just” disappear. f. Rinse the flask out immediately but take care not to get the X on the paper wet. g. Then put 40 cm3 of the 0.20 mol dm-3 sodium thiosulfate solution into the flask and add 10 cm3 of water. h. Add 5 cm3 of the hydrochloric acid and time as before. i. Repeat the experiment four more times using 30, 20, 15 and 10 cm3 of the thiosulfate solution making the total volume up to 50 cm3 with water each time. 2. Temperature. a. Measure 10 cm3 of 0.20 mol dm-3 thiosulfate solution. Add distilled water to a total volume of 50 cm3 into the conical flask and warm until the temperature is one or two degrees above 30-35 oC on a hot plate. b. Place the flask on the tile marked with a cross and add 5 cm3 of 2.0 mol dm-3 hydrochloric acid, timing as before. c. Record the temperature of the mixture after the acid has been added. d. Repeat the experiment using fresh portions of the 0.20 mol dm-3 thiosulfate solution each time at temperatures of approximately 40, 50 and 60 oC. FISK 2022 1 IBSL/HL Chem QUESTIONS: 1. Why do you think the flask should be rinsed immediately after each experiment? 2. Why is it important not to get the cross on the tile wet? 3. Using your results from experiment (1) plot a graph of molarity of the thiosulfate solution against time. Plot another graph of molarity against the reciprocal of time (1/t). 4. How does the rate of the reaction change with concentration? Suggest an explanation. 5. Using your results from experiment (2) plot a graph of time against temperature. Plot another graph of the reciprocal of time against temperature. 6. How does the rate of the reaction change with temperature? Suggest an explanation. 7. Apart from concentration and temperature, state two other factors which can influence the rate of a chemical reaction. PARTICLE SIMULATION 1. Go to https://www.explorelearning.com 2. Click on the “Enroll in a Class” button in the upper right hand corner of the web page 3. Type in the code 9NRTV3 to enroll in the class. 4. Click “Continue” and follow the directions on the site to complete your enrollment. 5. Once you are in the "IB Chemistry SL Year 1" class, click on the "Collision theory" link 6. Then click on "LAUNCH GIZMO" 7. Once the GIZMO is running - do what you need to do to appreciate this concept. You may choose to use the Student Exploration: Collision Theory worksheet along with the Gizmo simulation. 8. You may choose to work with a partner on this activity. 9. Complete the 5 multiple choice questions at the bottom of the gizmo when done. Student Submission: 1. Your Investigations Data 2. Data from a group who did the other investigation. 3. Answers to the Questions (do these on your own.) 4. Complete the 5 questions from the Gizmo. FISK 2022 2