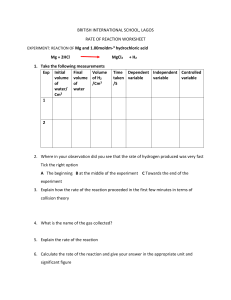
BRITISH INTERNATIONAL SCHOOL, LAGOS RATE OF REACTION WORKSHEET EXPERIMENT: REACTION OF Mg and 1.00moldm-3 hydrochloric acid Mg + 2HCl MgCl2 1. Take the following measurements Exp Initial Final Volume volume volume of H2 of of /Cm3 water/ water Cm3 1 + H2 Time Dependent Independent Controlled taken variable variable variable /S 2 2. Where in your observation did you see that the rate of hydrogen produced was very fast Tick the right option A The beginning B at the middle of the experiment C Towards the end of the experiment 3. Explain how the rate of the reaction proceeded in the first few minutes in terms of collision theory 4. What is the name of the gas collected? 5. Explain the rate of the reaction 6. Calculate the rate of the reaction and give your answer in the appropriate unit and significant figure