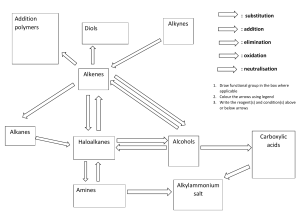
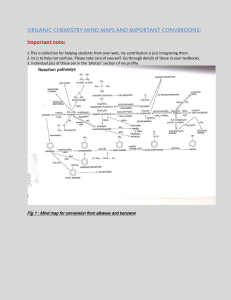
Delaware American School Class: Chemistry Students: Steven Umanzor Dennis Sánchez Teacher: Jorge Medina Date: 13/11/23 Functional Groups A functional group is what we call specific groupings of certain atoms within molecules that have their own characteristic properties. Here are some of the most commonly encountered functional groups. Note that “R” is a placeholder for a generic carbon substituent A second group of slightly less commonly encountered functional groups are here. This is by no means an exhaustive list, but it’s at least a good start. Another good way to get to know functional groups is to think about the relative electronegativities of the elements in each group. That will help you understand their properties and help you think about how they behave in chemical reactions. 1. Alkanes, Alkenes, Alkynes, and Aromatic Rings The hydrocarbon functional groups are very non-polar and tend to be extremely weak acids. In hydrocarbons, the only types of intermolecular interactions are London dispersion forces and their boiling points tend to be quite low, relative to molecules containing more polar functional groups. Alkanes are hydrocarbons containing no multiple bonds. Alkane substituents are called alkyl groups, which refers to alkanes lacking a C-H bond such as methyl, ethyl, or propyl. Common examples of alkanes are methane, ethane, propane, butane, and octane. The C-H bond is highly covalent and alkanes are very non-polar. They do not mix with water. Alkyl carbons are sp3 hybridized and have tetrahedral geometry about the carbon. You can often think of alkyl groups as the “spectator” functional groups of organic chemistry, abbreviated as R-. With the exceptions of free-radical substitution and, of course, combustion, alkanes don’t undergo a huge number of different reactions. They tend to provide the backbone of most organic molecules. Alkenes are hydrocarbons with one or more carbon-carbon double bonds. Common examples are ethene, propene, and butene. Alkene substituents are called alkenyl groups; vinyl is often used to refer to CH=CH2 . Alkenyl carbons are sp2 hybridized, with a trigonal planar geometry. Alkynes contain a carbon-carbon triple bond. Ethyne (acetylene) is the simplest alkyne; alkynes are sometimes called acetylenes. Alkyne substituents are called alkynyl groups. Terminal alkynes have a C-H. Internal alkynes have C-C bonds at each end. Alkynyl carbons are sp hybridized with a linear geometry. Benzene rings are six-membered rings containing 3 double bonds. Benzene rings are common in nature due to a property called aromaticity (nothing to do with its smell) that make them unusually stable. Can also be drawn as a hexagon with a circle. Benzene, methylbenzene (toluene) is responsible for the smell of model airplane glue. Benzene substituents C6H5– are called phenyl groups. The carbons in benzene are sp2 hybridized with trigonal planar geometry. 2. Alkanes, Alkenes, Alkynes, and Aromatic Rings The hydrocarbon functional groups are very non-polar and tend to be extremely weak acids.In hydrocarbons, the only types of intermolecular interactions are London dispersion forces and their boiling points tend to be quite low, relative to molecules containing more polar functional groups. Alkanes are hydrocarbons containing no multiple bonds. Alkane substituents are called alkyl groups, which refers to alkanes lacking a C-H bond such as methyl, ethyl, or propyl. Common examples of alkanes are methane, ethane, propane, butane, and octane. The C-H bond is highly covalent and alkanes are very non-polar. They do not mix with water. Alkyl carbons are sp3 hybridized and have tetrahedral geometry about the carbon. You can often think of alkyl groups as the “spectator” functional groups of organic chemistry, abbreviated as R-. With the exceptions of free-radical substitution and, of course, combustion, alkanes don’t undergo a huge number of different reactions. They tend to provide the backbone of most organic molecules. Alkenes are hydrocarbons with one or more carbon-carbon double bonds. Common examples are ethene, propene, and butene. Alkene substituents are called alkenyl groups; vinyl is often used to refer to CH=CH2 . Alkenyl carbons are sp2 hybridized, with a trigonal planar geometry. Alkynes contain a carbon-carbon triple bond. Ethyne (acetylene) is the simplest alkyne; alkynes are sometimes called acetylenes. Alkyne substituents are called alkynyl groups. Terminal alkynes have a C-H. Internal alkynes have C-C bonds at each end. Alkynyl carbons are sp hybridized with a linear geometry. Benzene rings are six-membered rings containing 3 double bonds. Benzene rings are common in nature due to a property called aromaticity (nothing to do with its smell) that make them unusually stable. Can also be drawn as a hexagon with a circle. Benzene, methylbenzene (toluene) is responsible for the smell of model airplane glue. Benzene substituents C6H5– are called phenyl groups. The carbons in benzene are sp2 hybridized with trigonal planar geometry. 3. Aldehydes, Ketones, Carboxylic Acids, Esters The C=O group is referred to as the carbonyl group. The C=O bond is strongly polarized towards oxygen and the carbon bears a partial positive charge. Carbonyls are found in aldehydes, ketones, esters, and carboxylic acids. Aldehydes RCHO have C=O bonded to carbon and to C-H Formaldehyde, acetaldehyde, and benzaldehyde are common examples. They have polar covalent bonding but are not hydrogen bond donors Ketones RC(O)R have C=O bonded to two carbons. Acetone (2-propanone) is nail polish remover. Carboxylic Acids RCOOH have a carbonyl bonded to -OH. They are distinct functional groups from alcohols. Acetic acid (vinegar) and formic acid are the simplest carboxylic acids. Other short-chain acids like butanoic and pentanoic acids are notorious for their locker-room smells (or worse). The hydroxyl group participates in hydrogen bonding and carboxylic acids have higher boiling points as a result. Despite the name, carboxylic acids tend to be relatively weak acids, not undergoing full dissociation in water (as compared to strong acids such as HCl and H2SO4). Esters RCOOR are similar to carboxylic acids, except the O-H bond is replaced with an O-C bond. Esters are notable for their sweet smells Contain polar bonds, but do not participate in hydrogen bonding. 4. Amides, Acid Halides, Anhydrides, Nitriles There are actually quite a few important functional groups containing carbonyls. These functional groups are all considered to be derived from carboxylic acids, as they can be obtained through replacement of OH with various groups. (Nitriles might not appear to be related to carboxylic acids at first glance, but they can actually be converted into amides through dehydration.) Amides contain a carbonyl carbon attached to an amino group. Amino acids linked together through formation of an amide are known as peptides. Amides containing N-H bonds can participate in hydrogen bonding. Acid Halides have -OH replaced with F, Cl, Br, or I. Anhydrides contain an oxygen flanked by two carbonyls. Distinct from esters. Anhydrides can be formed from two equivalents of a carboxylic acid with accompanying loss of H2O, hence the name. Nitriles don’t look like carboxylic acid derivatives at first, but they can be formed via the dehydration of amides. Acetonitrile is a common solvent. “Nitrile gloves” are made from nitrile rubber, a co-polymer of butadiene and acrylonitrile. The -CN substituent is sometimes referred to as a cyanide. HCN, hydrogen cyanide, is a highly toxic gas. The cyanide ion (-)CN, is often encountered in introductory courses and undergoes reactions with alkyl halides. 5. Miscellaneous: Epoxides, Thioethers, Nitro, Imine, Azide Technically, Epoxides are ethers, but since they participate in a number of reactions that ethers generally don’t , they deserve their own category. Thioethers (sulfides) are the sulfur equivalents of ethers. Dimethyl sulfide is the most commonly encountered example. Nitro groups are strongly electron-withdrawing. Nitromethane, a solvent, is the simplest example of a nitroalkane. Imines are the nitrogen-containing equivalents of aldehydes and ketones. Azides pop up from time to time. The “A” in the anti-HIV drug AZT stands for azido.