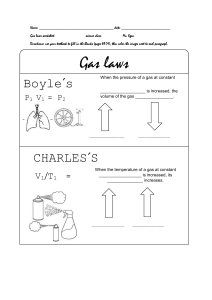
Detailed Lesson Plan in Science Grade 10 I. Objectives At the end of the lesson, the learners should be able to; Investigate the relationship between volume and pressure at a constant temperature of gas; Calculate simple problems using equations of Boyle’s Law Values Integration Relate Boyle’s Law to practical application in our daily lives. II. Topic and Lesson III. Boyle’s Law References: Science 10 (quarter 4 module 1) Materials: Laptop, visual aids, balloon, bottle with a hole on the side, graphing paper Procedure Teacher Activity A. 1. 2. 3. 4. 5. Pupil’s activity Preliminary Activities Greetings Prayer Checking attendance Classroom Protocol Review Fact or Bluff (Properties of gas) Say FACT If the properties being described is for GAS and BLUFF if not. 1. Gases expand the size of their container. NICE! 2. Gases flow easily. Very good! 3. Gases have a definite shape and volume. Great! 4. It has a low density Correct! 5. Particles of Gases move in random directions very fast traveling in a straight-line path. Very good! You do it well Science learner! FACT! FACT! BLUFF! FACT! FACT C. Motivation Using the balloon bottle activity ask the students the following question; 1. What causes the balloon to inflate? Very good! 2. What do you think will happen to the volume of the air in the balloon if we lessen the pressure inside the bottle? Correct! 3. What is the relation of pressure to the volume of air/gas inside the balloon? (Learner’s answer may vary ) All of your answers are great and it is all about our topic GAS LAW or also Known as Boyle’s Law . The presence of air or air pressure. If we lessen the pressure the volume will increases. As the pressure on the gas increases, the volume of the gas decreases because the gas particles are forced closer together. Conversely, as the pressure on the gas decreases, the gas volume increases because the gas particles now move farther apart. D. Lesson Proper Show the picture of Robert Boyle 1. Who is the man in the picture? Where did he become famous? Great! 2. Who is Robert Boyle Very Good! Robert William Boyle. Boyle’s Law Robert Boyle is an Irish chemist who carried out some of the earliest experiments that determined the quantitative relationship between the pressure and volume of a gas. 3. What are the two variables included in his Law? Correct! Pressure and Volume 4. What is the relationship of volume and pressure at constant temperature? Very well said! At a constant temperature, the volume of gas is inversely proportional to the pressure exerted by the gas. 5. What are the unit for volume and pressure? Great! Standard pressure 1 Atm = 760 torr 760mmHg 101.3kPa Standard for Volume 1 litter=1000m𝑥 2 1000ml 6. What is the mathematical equation for Boyle’s law? 7. How do you derive a formula from Boyle’s Law if the initial and final volume is missing? How do you derive a formula from Boyle’s Law if the initial or final pressure is missing? (Give sample problem) Steps to solve the problem: 1. Identify the given information and determine what is asked in the problem. 2. Write the equation. 3. Derive the equation to solve for an unknown quantity. 4. Substitute the known quantities into the equation, cancel the same unit copy the remaining unit, and calculate. 5. Think about the result whether it makes sense or not. Problem #1: A gas occupies 10 liters at a pressure of 40mmHg.What is the volume when the pressure is increased to 50mmHg? PV=K P1V1=P2V2 P1 = Initial pressure V1 =Initial Volume P2= Final Pressure V2= Final Volume Given: P1= 40mmHg V1= 10 liters P2=50mmhg V2=? P1V1=P2V2 P1V1=P2V2 P2 P2 40mmHg( 10 L)=50mmHg 400mmHg/L 50mmHg V2 =8.0 L Problem # 2: To what pressure must a gas be compressed in order to get into a 2.00 cubic foot tank is the entire weight of a gas that occupies 400.0 cu.ft.at standard pressure. Problem # 3: A gas occupies 1.5 L at 1.00 atm. What will be the volume of its gas if the pressure becomes 3.00 atm? Given P1= 1atm V1 = 400 cubic feet P2= ? V2= 2.0 cubic feet P1V1=P2 V2 V2 V2 1atm(400cu.ft.) P2 = 200 atm 2 Cubic feet Given: P1= 1atm V1=1.5L P2=3 atm V2=? 1atm( 1.5 L ) 3atm V2=0.5 L Problem #4 A gas occupies 12 L at 0.5 atm. What is the pressure if the volume becomes 5.0 L? Given: P1=0.5atm V1= 12L V2=5.0L P2=? 0.5 atm( 12 L) 5.0 L P2 = 1.2atm E. Generalization To sum it up what is the general knowledge that you learn from Boyle’s Law? Boyle’s law states that a volume of a gas is inversely proportional to pressure at a constant temperature. If the pressure increases the volume decreases and vice versa. Very good! F. Application In a real-life situation where can we apply Boyle’s Law Principle? IV.Evaluation Multiple Choice Direction: Read and answer each question/statement carefully. Identify what is being described. Encircle the letter of the correct answer. 1. According to Boyle’s Law, when the temperature is held constant, as pressure increases volume _______? a. Decreases c. increases b. Stay the same d. does not apply 2. Volume and temperature have a/an ____ Proportion. a. Direct c. inverse b. Equal d. cannot determine 3. Which of the following equation is for Boyle’s Law? a.P1/T1=P2/T2 c. P1V1=P2V2 b. V1n1=V2n2 d. V1/T1=V2/T2 4. Which of the following does Boyle’s Law apply? a. Bicycle tire b. hot air balloon c. Pressure Cooker d. none of the above a. Decreases d . Cannot determine c . P1V1=P2V2 a. Bicycle tire 5. A tank of He has an initial volume of 10mL at a pressure of 20atm. What would be the final volume of the gas if the pressure is increased by 30atm a. 5.67 mL c. 8.67mL b. 6.67 mL d. 10.6 mL VI.Assignment Answer the following questions. 1. What is Charles Law? 2. What are the variables included in this law? 3. What is the mathematical equation for Charles Law? Reference: Science 10 (Quarter 4-Module 1 ) Prepared by: Marisol M. Landayan b. 6.67mL