Uploaded by
JADHAV PRADIP SUBHANA KAMAL
IC Fabrication Technology: Semiconductors & Crystal Structures
advertisement

BITS Pilani
BITS Pilani
Pilani Campus
Sindhu S
Dept of Physics, BITS Pilani, Pilani Campus
Dept of EEE, WILP Division, Bangalore
MELZG 611
IC Fabrication Technology
Lecture No.3
Date . 05/08/2023
What is a Semiconductor?
Low resistivity => “conductor”
High resistivity => “insulator”
Intermediate resistivity => “semiconductor”
conductivity lies between that of conductors and
insulators
generally crystalline in structure for IC devices
In recent years, however, non-crystalline
semiconductors have become commercially
very important
polycrystalline
amorphous
crystalline
Why Si?
Ge:
Narrower bandgap (~0.6eV), leakier, Oxide unstable,
more expensive, less abundant.
Higher electron mobilities than silicon (higher speed).
Used only in select high performance applications with lower levels of
integration
GaAs:
Difficult to fabricate, higher defect densities, more expensive, difficult to
oxidize
Used extensively in optoelectronic applications
Si:
Abundant, inexpensive, easier processing, excellent quality of SiO2, ideal
physical and chemical properties, lower defects, etc.
4
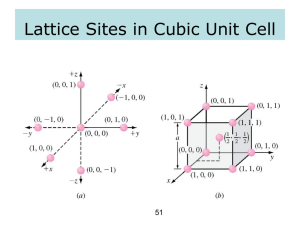
Crystallography and crystal structure
Crystals are described by their most basic structural element:
UNIT CELL.
Crystal: array of unit cells repeated in a regular manner over
3D.
Each edge of unit cell will have same length: cubic
symmetry.
Common crystalline structures: simple cubic, BCC and FCC
Unit cell
5
• An ideal crystal is a periodic array of structural
units, such as atoms or molecules.
• It can be constructed by the infinite repetition of these
identical structural units in space.
• Structure can be described in terms of a lattice, with
a group of atoms attached to each lattice point. The
group of atoms is the basis.
Bravais Lattice
An infinite array of discrete points with an arrangement
and orientation that appears exactly the same, from any of
the points the array is viewed from.
A three dimensional Bravais lattice consists of all points
with position vectors R that can be written as a linear
combination of primitive vectors. The expansion
coefficients must be integers.
Primitive Unit Cell
A primitive cell or primitive unit cell is a volume of
space that when translated through all the vectors in a
Bravais lattice just fills all of space without either
overlapping itself or leaving voids.
A primitive cell must contain precisely one lattice
point.
9
Crystals are described by their most basic structural
element: UNIT CELL.
Crystal: array of unit cells repeated in a regular manner
over 3D.
Each edge of unit cell will have same length: cubic
symmetry.
Common crystalline structures: simple cubic, BCC and
FCC
Unit cell
11
Unit cell types
Correspond to 6 distinct shapes, named after the 6 crystal systems
In each, representations include ones that are:
Primitive (P) – distance between layers is equal to the distance
between points in a layer
Body-centered (I) – extra point in the center
End-centered (A,B,C) – extra points on opposite faces, named
depending on axial relation
Face centered (F) – extra points at each face
13
Bravais Lattices
Assembly of the lattice points in 3-D results in 14
possible combinations
Those 14 combinations may have any of the 6 crystal
system (class) symmetries
These 14 possibilities are the Bravais lattices
c
c
c
b
a
b
b
P
P
a I
Monoclinic
a = g = 90o
abc
a
Triclinic
a g
abc
c
a
b
P
C
F
Orthorhombic
a = = g = 90o a b c
I
=C
c
c
a2
a1
a2
P
a1
I
Tetragonal
a = = g = 90o a1 = a2 c
P or C
R
Hexagonal
Rhombohedral
a = = 90o g = 0o a = = g 90o
a1 = a2 = a3
a1 = a2 c
a.k.a. Trigonal
a3
a2
a1
P
F
I
Isometric
a = = g = 90o a1 = a2 = a3
Simple Crystal Structures
There are several types of simple crystal structures:
Sodium Chloride (NaCl) -> FCC lattice, one
Na and one Cl atom separated by one half the
body diagonal of a unit cube.
Cesium Chloride -> BCC lattice with one
atom of opposite type at the body center
Hexagonal Closed packed structure (hcp)
Diamond structure -> Fcc lattice with
primitive basis that has two identical atoms
ZnS -> FCC in which the two atoms in the
basis are different.
Crystalline structure
Amorphous structure
Specifying
crystal
planes
:
Miller
Indices
Any of a set of three numbers or letters used to indicate
the position of a face or internal plane of a crystal and
determined on the basis of the reciprocal of the intercept of
the face or plane on the crystallographic axes.
Z
Z
Y
X
Y
X
(100)
Z
Y
X
(110)
(111)
MILLER INDICES
Directions
Planes
MILLER INDICES
Lattices
Crystals
Both are imaginary
Miller indices are used to specify directions and
planes:
These directions and planes could be in lattices
or in crystals:
The number of indices will match with the
dimension of the lattice or the crystal; in 1D
there will be 1 index and 2D there will be two
indices etc:
21
22
How to find the Miller Indices for an arbitrary direction? Procedure
Consider the example below
Subtract the coordinates of the end point from the starting point of the vector denoting the
direction If the starting point is A(1,3) and the final point is B(5,1) the difference
would be (4, 4)
Enclose in square brackets, remove comma and
write negative numbers with a bar [4 4]
Factor out the common factor 4[11]
If we are worried about the direction and
magnitude then we write 4[11]
If we consider only the direction then we
write [11]
Needless to say the first vector is 4 times
in length
The magnitude of the vector [11] = [11]
is (1) 2 (1) 2 = 2
24
Further points
General Miller indices for a direction in 3D is written as
[u v w]
The length of the vector represented by the Miller indices
is:
Notation Summary
•(h,k,l) represents a point
•Negative numbers/directions are denoted with a bar on
top of the number
•[hkl] represents a direction
•<hkl> represents a family of directions
•(hkl) represents a plane
•{hkl} represents a family of planes
Miller Indices for directions
•A vector r passing from the origin to a lattice point can
be written as:
r = r1a + r2b + r3 c
where, a, b, c → basic vectors and
miller indices → (r1r2r3)
Fractions in (r1r2r3) are eliminated by multiplying all
components by their common denominator.
[e.g. (1, ¾ ,½ ) will be expressed as (432)]
Index represents a set of all parallel vectos
Miller indices for planes
Consider the plane in
pink, which is one of an
infinite number of
parallel plane each a
consistent distance (“a”)
away from the origin
•The plane intersects the x-axis at point a.
It runs parallel along y and z axes.
•Thus, this plane can be designated as
(1,∞,∞)
Here the yellow plane
can be designated as
(∞,1,∞)
•And the green plane can
be written as (∞,∞,1)
• Miller Indices are the reciprocals
of the parameters of each crystal
face. Thus:
• Pink Face
= (1/1, 1/∞, 1/∞) = (100)
• Green Face
= (1/∞, 1/∞, 1/1) = (001)
• Yellow Face
= (1/∞, 1/1, 1/∞) = (010)
Miller indices here?
The plane of interest cuts two of the crystallographic axes.
• Intercepts: (1,1, ∞) → (110)
• This plane cuts all three crystallographic axes.
• Intercepts = (1,1,1) → (111)
This plane cuts two of the reference axes,
but not equidimensionally.
•Intercepts: (½, 1, 0) → (210)
Family of Directions
It’s a set of directions related by symmetry operations of the lattice.
Importance of Material Science
In Materials Science it is important to have a notation system
for atomic planes since these planes influence
•Optical properties
•Reactivity
•Surface tension
•Dislocations