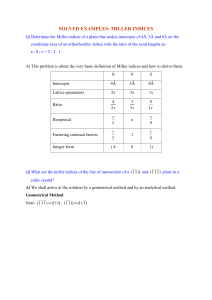
Mahavir Education Trust’s SHAH AND ANCHOR KUTCHHI ENGINEERING COLLEGE ENGINEERING PHYSICS-I JOURNAL Academic year : 2021-22 Experiment Number: Experiment-2 Title of the Experiment : Miller Indices Submitted by, Name of the student : Keshav Kumar Ramdhani Yadav Division : FE15 Roll Number : 52 Date of submission : 11-02-2022 EXPT.NO.2: Study of Miller Indices. AIM:- to study Miller Indices planes and direction APPARATUS:- Cubic unit cell models, thick paper, pasting material, ruler etc. FORMULA: Where, dhkl= is the interplanar distance between the planes with miller indices h,k,l. A=lattice constant THEORY: The crystal structure may be regarded as made up of an aggregate of a set of parallel equidistant planes known as crystal planes. The integers which determine the orientation of a crystal plane in relation to the three crystallographic axes are called Miller indices. Miller indices are the three smallest possible integers k and I which have the same ratios as the reciprocals of intercepts of the concerned plane on the three axes. The Miller indices for a particular crystal plane are determined in the following way. 1 First determine the intercepts of the plane on the three coordinate axes say p ,q and r. 2 Take the reciprocals of these numbers and by taking L.C.M, reduce them to the smallest three integers, as h, k. I such that h:k:l::1/p:1/q:1/r 3. The concerned plane is called to have Miller Indices (hkl) Miller Direction For cubic system normal to the plane (hkl) is the direction (hkl). The Miller direction for a particular crystal plane are determined in the following way. with an example [123] 1 Divide all three integers h,k,l by the highest number 2 At least one index will be unity 3. Select that particular axis as the vertical one. 4. Plot other two coordinates on remaining axes. 5. Find the point P(1/3,2/3,1) with coordinates obtained in step one. 6. Join that point P with the origin. Direction is OP. The inter planar spacing for a cubic system with lattice constant, is given by PROCEDURE ONLINE: 1. A value of x is given randomly to you by the system 2. You have to calculate y as per the formula given next to it and enter it into the box. Repeat same for value of z. 3. The value of x , y and z i.e. the side , face diagonal and body diagonal once calculated remain constant for the practical. 4. Select the crystal structure SC/BCC/FCC 5. For the given structure the values of internplannar distance for 100, 110 and 111 planes is given in terms of x,y,and z. You have to calculate the numerical values for interplanner distance and enter them into boxes given next to the respective interplanar distances. 6. Once you have entered the interplanar distance for all three set of planes, click on calculate. 7. This calculates the practical ratio of interplanar distance for three set of above mentioned planes. 8. Just try to verify if the calculated ratio is equal to theoretical ration just mentioned above the table. Answer (Yes/No). DO NOT SUBMIT. Just take screen shot of the table and insert in the position of empty table put below: 9. Repeat the above procedure for BCC and FCC structure. 10. SUBMIT NOW 11. You will be taken to Miller Practise session. You can draw Miller planes using instructions given on screen or by hand and submit with this practical. PROCEDURE OFFLINE: 1.Lattice constant (a=__) will be provided from the lab to every student. 2. Construct a cube of side a using the sheet given. 3. Measure the edge of the cube (x=___), Face diagonal of the cube (y=__) and the body (z=__) 4. Identify planes (100), (110) & (111) in SC, BCC and FCC unit cell models. 5. Determine interplanar distance d100, d110 & d111. 6. Find their ratio and compare it with theoretical values. 7. Draw the crystal planes (100),(110),(111),(120)&(103) and the directions [110],[212],[021],[301] and[001]. OBSERVATION TABLE FOR SC: - OBSERVATION TABLE FOR BCC: - OBSERVATION TABLE FOR FCC: - MILLER INDICES: - (011) (222) (010) (111) (110) RESULT:- The theoretical and experimental values are in agreement. Evaluation Table 4: Name student of Keshav Yadav Name of Miller Indices Experiment Division -> FE15 Roll NO -> 52 Attendance & Execution (04) Documentation & timely submission (03) Viva (03) Total (10)