The Future of Traceability The Impact of eQMS on Electronic Batch Records
advertisement

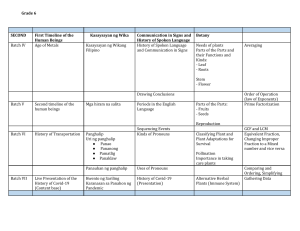
SOLUTIONS WHY C15? RESOURCES NEWS & BLOG COMPANY PRICING CONTACT US Schedule a Demo News & Blog Case Studies & ROI Contracts Environmental & Social Governance GMP Risk Governance & Product Liability Supply Chain The Future of Traceability: The Impact of eQMS on Electronic Batch Records Traceability is a fundamental element in ensuring product quality, safety, and compliance. Whether it's cannabis, pharmaceuticals, or food and beverages, being able to track and document the entire production process is essential. With advancements in modern technology, traditional paper-based systems are gradually giving way to more sophisticated and efficient solutions. One revolutionary development in this realm is the adoption of Electronic Batch Records powered by electronic Quality Management Systems (eQMS). What are Batch Records? A batch record serves as the life history of a product batch, documenting all processes and steps it undergoes to become the final product. It includes essential information such as dates, growth stage transitions, location changes, nutrient application, Integrated Pest Management (IPM) tasks, and all cultivation and processing steps outlined in Standard Operating Procedures (SOPs). It also must include any quality-related issues during the production process whether there was an unplanned deviation, an issue with input (raw) materials, or even testing of the final product (i.e. an out-of-spec Certificate of Analysis or ‘COA’). For a robust quality assurance program, it is crucial to ensure that batch records are exceptionally detailed. However, creating such detailed batch records demands significant time and effort from both Quality Assurance and Operations teams, potentially causing delays in batch release and overall production timelines. QMS streamlines these efforts without SOLUTIONS WHY C15? NEWS & BLOGcompromising COMPANY quality or compliance. Plus, without linking your executed batch records to the Schedule a Demo master batch record in your library,RESOURCES there is also aPRICING risk of not CONTACT referencingUSthe correct version. The Evolution of Batch Records: From Paper to Electronic For decades, industries have relied on paper-based batch records to document the step-by-step processes involved in manufacturing products. While these records served their purpose, they were not without limitations. Paper-based systems were time-consuming, error-prone, and challenging to maintain, especially in companies dealing with high production volumes. The emergence of electronic systems began to transform these challenges into opportunities. eQMS offers a centralized platform for document control, training, deviations, and other quality processes - becoming a game-changer in terms of traceability! C15 Solutions consolidates key information such as sanitation records, quality investigations, COA results, supplier corrective action requests (‘SCARs’) and equipment calibration records. The platform’s Automated COA Analysis provides an instant pass or fail result, accompanied by a visual trend analysis that facilitates the batch-release process, ensuring conformity with target specifications. Ensure production readiness with visibility into your equipment status. Learn how else C15 is empowering cannabis businesses. How eQMS and Electronic Batch Records Revolutionize Manufacturing Real-Time Data Capture Immediate visibility into operations enables companies to identify and rectify issues promptly. Decision-makers can access live data, enabling a faster response to potential problems and ensuring product quality remains uncompromised. SOLUTIONS WHY C15? RESOURCES NEWS & BLOG COMPANY PRICING CONTACT US C15 gives you real-time visibility into the bottlenecks across your operation. Reduced Risk of Errors Manual data entry often leads to errors. eQMS eliminates this risk by governing the entire batch run with stage-gated workflows, thereby automating data capture, minimizing human intervention, and maintaining data integrity. Enhanced Compliance In highly regulated industries, the U.S. Food and Drug Administration's (FDA) Current Good Manufacturing Practices (cGMP) require proof of proper handling for every step of the production process. Batch records and other types of manufacturing documentation demonstrate this level of accountability. eQMS allows companies to create standardized workflows and enforce compliance across the organization, making audits smoother and less timeconsuming. Moreover, eQMS offers compliant e-signatures, a requirement for 21 CFR Part 11 compliance (ONLY eQMS offers this feature - ERP, S2S & MES platforms do NOT offer compliant e-signatures). Interconnected Systems eQMS platforms integrate with other critical systems like environment monitors/controls, Enterprise Resource Planning (ERP) platforms, and Laboratory Information Management System (LIMS) platforms. This interconnectedness ensures a seamless flow of information across the organization, enabling better collaboration between departments. Version Control and Audit Trails Keeping track of changes to batch records is crucial for maintaining an accurate historical record. eQMS offers robust version control and audit trail capabilities, allowing users to view previous versions, track changes, and understand the sequence of events during the manufacturing process. Data Analytics and Continuous Improvement When you have your batch-run process handled by your eQMS, the production team gets visibility across the entire operation. Relevant SOPs are associated with each production process and equipment calibration and status can be Schedule a Demo verified pre-run. Batch-runs can be linked to real-time quality investigations and processed accordingly. SOLUTIONS WHY C15? NEWS & BLOG COMPANY Schedule a Demo RESOURCES PRICING Plus, by leveraging analytics, companies can enhance overallCONTACT efficiency,US resulting in cost savings and higher-quality products! C15 has developed an eQMS platform that enables companies to reduce their cost of quality by 3040% To give further context into how large those savings are, they typically represent anywhere from 10-20% of overall operating expenses - based on actual C15 customer ROI analyses. Above & beyond automated pass/fail analysis, C15 allows you to trend COA results. Training Management & Competency An eQMS platform can track employee training and competencies, ensuring that personnel involved in the batch-run process are adequately trained and qualified for their respective roles. Mastering the Digital Shift Companies that embrace electronic systems and leverage their benefits will position themselves at the forefront of quality management but there are some considerations that companies must address during implementation. Initial Investment: Transitioning from a paper-based system to eQMS requires an initial investment in technology, training, and integration. However, the longterm benefits outweigh the upfront costs. Data Security: As data becomes digitized, ensuring its security and protection against unauthorized access becomes paramount. User Training and Adoption: Proper training and change management is crucial to ensuring that employees embrace the new electronic systems. User buy-in and adoption are essential for the success of any eQMS implementation. Regulatory Compliance: While eQMS can enhance compliance, companies must ensure that electronic systems meet regulatory standards such as 21 CFR Part 11. SOLUTIONS WHY C15? NEWS & BLOG COMPANY Schedule a Demo PRICINGof eQMS CONTACT US The future of traceability lies in theRESOURCES seamless integration and Electronic Batch Records as you can eliminate paper from the line with industry-leading technology. Looking to maintain a sustainable, competitive edge? Book a demo today. < Older Post Newer Post > Share Link: Simplified Compliance & Quality Control for Cannabis QUICK LINKS Quality Management System Why C15? Company Support Portal Resources Schedule a Demo CONTACT INFORMATION Feel free to get in touch with us by phone or send us an e-mail. General Enquiries: 1-855-507-1589 info@c15solutions.com Direct: 1-416-543-2496 Schedule a Demo OUR LOCATION 2902 South Sheridan Way, Suite 203, Oakville, Ontario, Canada L6J 7L6 SOLUTIONS scott.samuel@c15solutions.com WHY C15? NEWS & BLOG COMPANY Technical Support: PRICING CONTACT US RESOURCES support@c15solutions.com © 2023 C15 Solutions, Inc. | Website by KUSHY Schedule a Demo