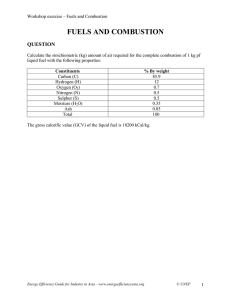
MATERIAL BALANCE WITH CHEMICAL From roll no: REACTIONS TOPICS Introduction 4.2 Principles of stoichiometry 4.2.1 Stoichiometric Coefficient 4.2.2 Stoichiometric Ratio 4.2.3 Stoichiometric Propotion 4.2.4 Limiting Reactant TOPICS 3 4.2.5 Excess Reactant 4.2.6 Percentage of Excess Reactant 4.2.7 Inerts 4.2.8 Percentage Conversion 4.2.9 Percentage Yield 4.2.10 Selectivity 4.3 Material Balance with single Chemical Reactions 4.4 Material Balance with Multiple Chemical Reactions 4.5 Combustion of Fuels 4.5.1 Introduction 4.5.2 Solid fuels 4.5.3 Liquid Fuels 4.5.4 Gaseous Fuels 4.5.5 Calorific value of Fuels 4.6 Analysis of fuel 4.6.1 Proximate Analysis 4.6.2 Ultimate analysis 4.6.3 analysis of flue gas. Orsat Analysis and Wet basis 4.7 Solved Examples 4.8 Exercises INTRODUCTION Material balances are important first step when designing a new process or analyzing an existing one. • Material balances are nothing more than the application of the law of conservation of mass, which states that mass can neither be created nor destroyed. • They are used in industry to calculate mass flow rates of different streams entering or leaving chemical or physical processes Presentation title 5 THE GENERAL BALANCE EQUATION Suppose propane is a component of both the input and output streams of a continuous process unit shown below, these flow rates of the input and output are measured and found to be different Input + generation − output − consumption = accumulation Q… Presentation title PROCEDURE FOR MATERIAL BALANCE CALCULATIONS. • Draw and label the process flow chart (block diagram). When labeling, write the values of known streams and assign symbols to unknown stream variables. Use the minimum number possible of symbols. • Select a basis of calculation. This is usually the given stream amounts or flow rates, if no given then assume an amount of a stream with known composition. • Write material balance equations. Note in here the maximum number of independent equations you can write for each system is equal the number of species in the input and output streams of this system. Also note to first write balances that involve the fewest unknown variables 6 7 “ BALANCES ON REACTIVE SYSTEMS • Theory of proportions in which chemical compounds react is called stoichiometry. • A statement of the relative number of moles or molecules reacting to produce products is given by a chemical equation known as stoichiometric equation. Presentation title • For example, 2 moles of SO2 and one mole of O2 react to produce 2 moles of SO3. Then the stoichiometric equation will be 2SO2+O2→2SO3. Numbers that precede the formulas are known as stoichiometric coefficients. • In a stoichiometric equation, the number of atoms in both sides of the equation must be balanced. Ratio of stoichiometric coefficients of two species is known as stoichiometric ratio • EXAMPLE: 2SO2+O2→ 2SO3. What is the stoichiometric ratio of SO2 to SO3? • Solution stoichiometric ratio of SO2 to SO3 =2 mole of SO reacted/2 mole of SO produced 2/2 = 1 8 Presentation title 9 LIMITING & EXCESS REACTANTS • The chemical compound which is present less than its stoichiometric amount, will disappear first. This reactant will be the limiting reactant and all the others will be excess reactants. • Ex: 2SO2+O2→ 2SO3 Presentation title YIELD, SELECTIVITY&CONVERSION 10 Presentation title Combustion is a chemical process in which a substance reacts rapidly with oxygen and gives off heat. The original substance is called the fuel, and the source of oxygen is called the oxidizer. The fuel can be a solid, liquid, or gas. Wood, LPG, Kerosene, coal, petrol, natural gas, diesel, biogas etc. are some fuels used in our daily lives. Different types of fuel produce different amounts of heat on combustion or on burning. Complete step by step answer: We all use LPG, Kerosene, coal, petrol, natural gas, diesel, biogas in our daily lives. The heat energy that is produced by the combustion or burning of fuel can be used to cook food, running factory machines and motor vehicles and can be converted to electrical energy. Based on state, fuels can be classified into three categories as solid fuels, liquid fuels and gaseous fuels. Solid fuel: Coal and wood are solid fuels. Coal is used in industries and factories while wood is used mostly in homes. Liquid fuel: kerosene and petrol are liquid fuels. Kerosene is used in stoves to cook food while petrol is used in vehicles. • • • • • • • COMBUSTION OF FUELS 11 Presentation title 12 Gaseous fuel: CNG and LPG are gaseous fuels. LPG is used in homes and in vehicles while CNG is used to drive vehicles. Ideal fuel should have high calorific value, burn easily at moderate rate in air, must have proper ignition temperature, should not produce harmful gases on combustion, fuel should be cheap, easy to transport etc. calorific value: The amount of heat that is produced by the complete combustion or burning of 1 kg of fuel is termed as its calorific value expressed in kilojoules per kilogram. The calorific value for kerosene is 45,000 KJ/Kg which means 1kg kerosene is completely burned, it produces 45,000 KJ of heat. 13 ANALYSIS OF FUEL PROXIMATE ANALYSIS • • • proximate explanations are concerned with how it works. Proximate analysis includes moisture content, volatile matter, and fixed carbon. The proximate analysis of coal separates the products into four groups: (1) moisture, (2) volatile matter, consisting of gases and vapors driven off during pyrolysis, (3) fixed carbon, the nonvolatile fraction of coal, and (4) ash, the inorganic residue remaining after combustion. ULTIMATE ANALYSIS • • • ultimate explanations are concerned with why a behavior exists. ultimate analysis is carbon, hydrogen and oxygen. The ultimate analysis of coal involves determination of the weight percent carbon as well as sulfur, nitrogen, and oxygen (usually estimated by difference). Trace elements that occur in coal are typically included as a part of the ultimate analysis. The standard method for the ultimate analysis of coal and coke includes the determination of elemental carbon, hydrogen, sulfur, and nitrogen, together with the ash in the material as a whole. 14 TYPES OF FLUE GAS ANALYSIS FLUE OR STACK ORSAT ANALYSIS OR Orsat analysisBASIS or dry basis: all the gases resulting from a Flue or stack gas: all GAS DRY combustion process not including the water vapor. (Orsat the gases resulting from a combustion process including the water vapor, sometimes known as a wet basis. analysis refers to a type of gas analysis apparatus in which the volumes of the respective gases are measured over and in equilibrium with water; hence each component is saturated with water vapor. The net result of the analysis is to eliminate water as a component that is measured.) Look at Figure below. To convert from one analysis to another, you have to adjust the percentages of the components to the desired basis.