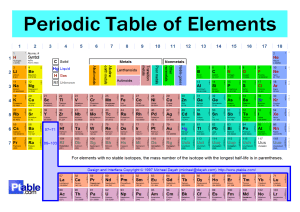
Cambridge IGCSE Chemistry Topic 9: The Periodic Table Noble gases Notes www.pmt.education Describe the noble gases, in Group VIII or 0, as being… ● Unreactive, monatomic gases (exist as He rather than He2) ● They have 8 electrons in their outer shell (except helium, which has 2). ● They are unreactive and do not easily form molecules, because they have full outer shells, meaning they have a stable arrangement of electrons. State the uses of the noble gases in providing an inert atmosphere, i.e. argon in lamps, helium for filling balloons ● Helium- filling balloons: less dense than air so the balloons float and He is non-flammable ● Neon – advertising signs ● argon/krypton/xenon- gas in filament lamps: inert ● argon- shielding gas in welding: denser than air so keeps air off of metal and inert so metal won’t oxidise www.pmt.education Cambridge IGCSE Chemistry Topic 9: The Periodic Table Transition elements Notes www.pmt.education Describe the transition elements as… ● A collection of metals having high densities, high melting points and forming coloured compounds, and which, as elements and compounds, often act as catalysts (Extended only) Know that transition elements have variable oxidation states ● Transition elements have variable oxidation states, meaning they can form ions with different charges o E.g. Cu+ or Cu2+ ● the oxidation state a transition metal is in is often shown using roman numerals e.g. iron (II) = Fe2+ and iron (III) = Fe3+ www.pmt.education Cambridge IGCSE Chemistry Topic 9: The Periodic Table Group properties Notes www.pmt.education Describe lithium, sodium and potassium in Group I as… ● A collection of relatively soft metals showing a trend in melting point, density and reaction with water (Extended only) Identify trends in Groups, given information about the elements concerned ● Similar chemical properties due to the fact that they have the same number of outer shell electrons ● Once you are given information regarding more than one element in a group, look at each of their positions in the group (i.e. near the top or bottom) and identify the trend shown by the elements with given information (e.g. reactivity or boiling point could increase down the group) o Going down a group means going up in number of electron shells, more electron shielding and so less attraction between the nucleus and outer shell electrons Predict the properties of other elements in Group I, given data, where appropriate ● Melting point o Low melting points compared to most other metals ▪ As you go down the group, melting points decrease ● Density o Low densities – they will float on water ▪ As you go down the group, densities increase ● Reaction with water o All react vigorously with water to create an alkaline solution and hydrogen (i.e. you will see bubbling/effervescing due to the production of a gas) o More bubbles with reaction with water = more vigorous reaction = more reactive alkali metal (Group I metal) ▪ Reactivity increases down the group (so reaction becomes more vigorous) ▪ Down the group – easier to lose electrons and form positive metal ions (cations), these are formed when metals react ▪ It is easier to lose electrons due to the increase in electron shells and therefore there is more electron shielding and easier to lose electrons due to the decrease in attraction between the positively charged nucleus and the negatively charged outer shell electrons www.pmt.education Describe the halogens, chlorine, bromine and iodine in Group VII, as… ● A collection of diatomic non-metals showing a trend in colour and density … and state their reaction with other halide ions ● reactivity decreases going down the group: ○ outer shell becomes further from nucleus and there is more shielding from inner electrons ○ attraction between nucleus and outer electrons decreases ○ electrons are gained less easily (which is how halogens react) ● a more reactive halogen will displace halide ions of a less reactive halogen ● chlorine will displace both bromide and iodide ions: ○ chlorine + sodium bromide → sodium chloride + bromine ○ chlorine + sodium iodide → sodium chloride + iodine ● bromine will displace iodide but not chloride ions ○ bromine + potassium iodide → potassium bromide ● iodine will not displace chloride or bromide ions Predict the properties of other elements in Group VII, given data where appropriate ● Colour o Darker in colour as you go down the group ▪ Fluorine is very pale yellow ▪ Chlorine is yellow-green ▪ Bromine is red-brown ▪ Iodine is purple ● Density o Density increases as you go down the group ▪ Chlorine is a gas ▪ Bromine is a liquid ▪ Iodine is a solid ● reactivity o decreases down the group o outer shell becomes further from nucleus and there is more shielding from inner electrons, attraction between nucleus and outer electrons decreases, electrons are gained less easily (which is how halogens react) o A more reactive halogen can displace a less reactive one in an aqueous solution of its salt www.pmt.education Cambridge IGCSE Chemistry Topic 9: The Periodic Table Periodic trends Notes www.pmt.education Describe the change from metallic to non-metallic character across a period ● From left to right elements change from metallic to non-metallic character ● Metallic character/properties: o Shiny o Conductive o Dense o Malleable ● On either side of the red line, you have some elements known as “metalloids” that have both metallic and nonmetallic properties, such as silicon (which forms silicon dioxide…) (Extended only) Describe and explain the relationship between Group number, number of outer shell electrons and metallic/non-metallic character ● Group number- shows the number of electrons in the outer shell ● Metallic / nonmetallic- metals form positive ions by losing electrons and nonmetals form negative ions by gaining electrons. All of group 1 and 2 are metals, all of group 7 and 8 (0) are nonmetals. In groups 3,4,5,6 there is a transition between metals and nonmetals. www.pmt.education Cambridge IGCSE Chemistry Topic 9: The Periodic Table The Periodic Table Notes www.pmt.education Describe the Periodic Table as a method of classifying elements and its use to predict properties of elements ● The Periodic Table can be used to classify elements and predict properties of elements by the way that they are arranged in the table… o Elements are arranged in order of atomic (proton) number (bottom number) and so that elements with similar properties are in columns, known as groups. o Elements in the same periodic group have the same amount of electrons in their outer shell, which gives them similar chemical properties. ● You can deduce the electronic configurations of elements from their positions in the Periodic Table o Group 1 has 1 electron in its outer shell, group 2 has 2 etc… o Period 1 has 1 shell, period 2 has 2 shells etc… www.pmt.education