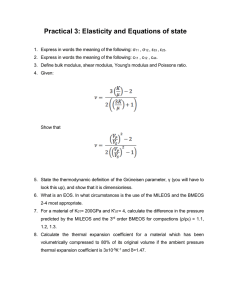
THERMODYNAMICS The study of HEAT and its transformation into mechanical energy. Greek Word: "therme" - heat "dynamis" - power THERMODYNAMIC SYSTEMS SYSTEM SURROUNDINGS BOUNDARY The part of the surroundings that we chose to study. The rest of the universe. The surface dividing the system and surroundings. KINDS OF THERMODYNAMIC SYSTEMS OPEN CLOSED ISOLATED Mass and Energy can transfer between the system and the surroundings. Energy can transfer between the system and the surroundings, but not mass. Neither Mass nor Energy can transfer between the system and the surroundings. PROPERTIES OF A SYSTEM A property is any quantity, which serves to describe a system. INTENSIVE EXTENSIVE Is one which does not depend on the size of the system such as temperature, pressure, density, and velocity. Is one which depends on the size of the system such as volume, momentum, and kinetic energy. TEMPERATURE "hotness" or "coldness" of a body a measure of the kinetic energy of the particles in a sample of matter. Absolute Zero is the temperature at which the molecules stop moving. It is equivalent to 0 K. Absolute Temperature is the temperature measured from absolute zero. TEMPERATURE CONVERSION Relationship between Celsius and Kelvin Relationship between Fahrenheit and Rankine Relationship between Celsius and Fahrenheit PROPERTIES OF A SUBSTANCE PRESSURE Is the force exerted per unit area. DENSITY Is the mass per unit volume. SPECIFIC VOLUME Is the volume per unit mass. SPECIFIC WEIGHT Is the weight per unit volume SPECIFIC GRAVITY/ RELATIVE DENSITY Is the ratio of the density of a certain substance to the density. PROPERTIES OF A SUBSTANCE PRESSURE continuous physical force exerted on or against an object by something in contact with it. SI Unit: Pascal (Pa) which is equivalent to Newton per square meter (N/m^2) 101,325 Pa = 101.325kPa = 1 atm = 760 torr = 760mmHg = 14.7 psi (pound force per square inch) = 1.013x10^6 dyne/cm^2 = 1.013 bar PROPERTIES OF A SUBSTANCE DENSITY p = Density (kg/m^3) m = mass (kg) V = Volume (m^3) PROPERTIES OF A SUBSTANCE SPECIFIC VOLUME v = Specific Volume (m^3/kg) p = Density (kg/m^3) m = mass (kg) V = Volume (m^3) PROPERTIES OF A SUBSTANCE SPECIFIC WEIGHT W = Weight (N or kgm/s^2) p = Density (kg/m^3) m = mass (kg) V = Volume (m^3) g = acceleration due to gravity (9.8m/s^2) PROPERTIES OF A SUBSTANCE SPECIFIC GRAVITY/REALTIVE DENSITY sg_substance = Specific Gravity of a substance with respect to reference (unitless) p_substance = Density of the substance (kg/m^3) p_H2O = Density of Water (1,000kg/m^3 or 1g/cm^3) PROBLEM #1 The air pressure for a certain tire is 109 kPa. What is this pressure in (a) atm (b) torr (c) mmHg (d) pound per square inch - psi (e) dyne/cm^2 (f) bar PROBLEM #2 A certain rectangular material weighs 45g with 3cm height, 2.5cm width and 2cm length. Find the density and its specific volume counterpart (a) in g/cm^3 (b) in g/mL (c) in kg/m^3 (d) in kg/L (e) in slug/ft^3 (f) in lbm/in^3 PROBLEM #3 A cylinder of plastic is 100 mm long, and 50 mm in diameter. It has a mass of 1 kg. Determine its specific gravity and indicate whether it would float or sink in water. PROBLEM #4 A piece of unknown material has an intricate shape. It has a mass of 126 g. You submerge it to find it displaces 422 ml of water. What is the specific gravity of the piece? LAWS OF THERMODYNAMICS 0th Law Equivalent Relation 1st Law Conservation of Energy 2nd Law State of Disorder 3rd Law Absolute Zero 0TH LAW EQUIVALENT RELATION "If 2 objects are separately in thermal equilibrium with third object, then the two objects are in thermal equilibrium with each other." THERMAL EQUILIBRIUM This phenomenon is obtained if two objects with different temperature is made to have contact, and reach a common temperature. In thermal equilibrium, the transfer of heat stopped. THERMAL EXPANSION As temperature increases, dimensions of objects increases. ΔL = Change in Length (m) α = Coefficient of Thermal Linear Expansion (/℃) γ = Coefficient of Area Thermal Expansion (/℃) β = Coefficient of Volume Thermal Expansion (/℃) Lo = Original Length (m) Ao = Original Surface Area (m^2) Vo = Original Volume (m^3) ΔT = Change in Temperature (℃) Coefficient of Thermal Expansion on Various Materials MATERIAL Aluminum Brass Brick or Concrete Copper Glass, Pyrex COEFFICIENT OF THERMAL EXPANSION (α: /℃) MATERIAL COEFFICIENT OF THERMAL EXPANSION (α: /℃) Glass, Window 9.0 x 10^-6 Gold 14 x 10^-6 Ice 52 x 10^-6 Iron/Steel 12 x 10^-6 24 x 10^6 20 x 10^-6 12 x 10^-6 17 x 10^-6 3.3 x 10^-6 Coefficient of Thermal Expansion on Various Materials MATERIAL COEFFICIENT OF THERMAL EXPANSION (β: /℃) MATERIAL COEFFICIENT OF THERMAL EXPANSION (β: /℃) Ethyl Alcohol 1.1 x 10^-4 Mercury 1.8 x 10^-4 Gasoline 9.5 x 10^-4 Water 2.1 x 10^-4 Glycerin 4.9 x 10^-4 Air 3.5 x 10^-3 PROBLEM #5 A surveyor uses a steel measuring tape that is exactly 50.000m long at a temperature of 20℃. (a) What is its length on a hot summer day when the temperature is 35℃. (b) The surveyor uses the measuring tape to measure a distance when the temperature is 35℃. The value he reads off the tape is 35.794. What is the actual distance? Assume that the tape is calibrated for use at 20℃. PROBLEM #6 A glass flask with volume 200cm^3 is filled with to the brim with mercury at 20℃. How much mercury overflows when the temperature of the system is raised to 100℃? The coefficient of linear expansion of the glass is 0.40x10^-5 /K. PROBLEM #7 The outer diameter of a glass jar and the inner diameter of its iron lid are both 725mm at room temperature (20.0℃). What will be the size of the mismatch between the lid and the jar if the lid is briefly held under hot water until its temperature rises to 50.0℃, without changing the temperature of the glass? STRESS STRAIN characterizes the strength of the forces causing thr deformation. describes the resulting deformation σ = Tensional Stress (N/m^2) F = Tension (N) A = Area (m^2) ε = Strain ΔL = Change in Length (m) Lo = Original Length (m) MODULUS OF ELASTICITY The proportionality constant of the stress and strain. Y = Modulus of Elasticity ΔL = Change in Length (m) Lo = Original Length (m) Modulus of Elasticity of Various Materials MATERIAL MODULUS OF ELASTICITY (GPa) MATERIAL MODULUS OF ELASTICITY (GPa) Aluminum 70 Steel 200 Brass 90 Lead 16 Iron 210 Nickel 210 Copper 110 Concrete 40 Glass 60 THERMAL STRESS The compression or expansion of material due to change in temperature. Y = Modulus of Elasticity ΔT = Change in Temperature (℃) α = Coefficient of Thermal Linear Expansion (/℃) σ_thermal = Thermal Stress PROBLEM #8 An aluminum cylinder 10cm long, with a crosssectional area of 20cm^2, is to be used as a spacer between two steel walls. At 17.2℃ it just slips in between the walls. When it warms to 22.3℃, calculate the stress in the cylinder and the total force it exerts on each wall, assuming that the walls are perfectly rigid and a constant distance apart. PROBLEM #9 A brass rod is 185cm long and 1.60cm in diameter. What force must be applied to each end of the rod to prevent it from contracting when it is cooled from 120.0℃ to 10.0℃? PROBLEM #10 Steel train rails are laid in 12.0m long segments placed end to end. The rails are laid on a winter day when their temperature is -2.0℃. (a) How much space must be left between adjacent rails if they are just to touch on a summer day when their temperature is 33.0℃? (b) If the rails are originally laid in contact, what is the stress in them on a summer day when their temperature is 33.0℃? Defined as the transfer of energy across the boundary of a system due to a temperature difference between system and surrounding. Calorie (cal) - the amount of heat required to raise 1℃ temperature of a 1 gram of water. HEAT British Thermal Unit (BTU) - is the quantity of heat required to raise 1℉ of 1lb of water. 1cal = 4.186J 1BTU = 1,054J SPECIFIC HEAT Denoted as c It is a measure of how thermally sensitive or insensitive a substance is to the addition of energy. Heat Capacity per unit mass. Q = Heat m = mass c = specific heat ΔT = Change in Temperature MOLAR HEAT CAPACITY Denoted as C A proportionality constant between heat and the temperature change. Q = Heat C = Molar Heat Capacity ΔT = Change in Temperature M = Molar Mass n = number of moles Specific Heats of materials (Constant Pressure) MATERIAL Specific Heat (J/kg K) Molar Mass, M (kg/mol) Aluminum 910 0.0270 Water (liquid) 4160 0.018 Ice 2100 0.018 Copper 390 0.0635 Salt (NaCl) 879 0.0585 Iron 470 0.055 LATENT HEAT The heat required per unit mass to change the Internal Energy without changing the temperature in order to undergo phase change. n io tio su tio n bl sa in en at nd at de or po ap si n ev io n Q = Heat (J) m = mass (kg) L = Latent Heat (J/kg) Latent Heat of Vaporization Lv (H2O) = 2,256.7kJ/kg co Latent Heat of Sublimation Ls (H2O) = 2838 kJ/kg melting freezing Latent Heat of Fusion Lf (H2O) = 333.5 kJ/kg PROBLEM #11 A man working in the field drinks hot water out of an aluminum cup. The cup has a mass of 0.120kg and is initially at 20.0℃ when she pours in 0.300kg of water initially at 70.0℃. What is the final temperature after the water and the cup attain thermal equilibrium? PROBLEM #12 An aluminum tea kettle with mass 1.50kg and containing 1.80kg of water is placed on a stove. If no heat is lost to surroundings, how much heat must be added to raise the temperature from 20.0℃ to 85.0℃? PROBLEM #13 A physics student wants to cool 0.25kg of water initially at 25.0℃, by adding ice initially at -20℃. How much ice should she add so that the final temperature will be 0℃ with all the ice melted if the heat capacity of the container may be neglected. PROBLEM #14 A heavy copper pot of mass 2.0kg (including the copper lid) is at a temperature of 150℃. You pour 0.10kg of water at 25℃ into the pot, then quickly close the lid of the pot so that no steam can escape. Find the final temperature of the pot and its contents, and determine the phase (liquid or gas) of the water. Assume that no heat is lost to the surroundings.