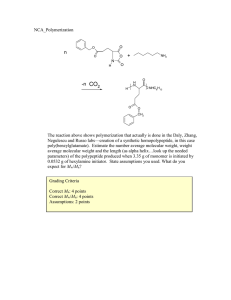
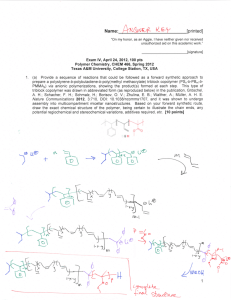
CHME 435 – Mid Term Exam Fall 2009/2010 SOLUTION 1. (2 pts) Plot qualitatively density versus temperature for a semicrystalline polymer. Show and name any transition temperature (such as Tg and/or Tm) on the plot. Density Tg Tm T 2. (4 pts) In the following table, list four types of polymerization processes, list the materials/chemicals involved in each, give the solubility information in case a solvent/water is used (what is/are soluble in solvent/water and the level of solubility and what is/are not soluble) and briefly give the advantages and disadvantages the process as compared with the others (especially regarding heat removal and viscosity increase). Polymerization Processes Materials/ Chemicals Involved Solubility Information Advantages/Disadvantages and Comparison with Other Processes Bulk Polymerization Monomer Initiator Polymer Polymer might be soluble in monomer. Heat removal to prevent boiling of monomer is difficult. Solution Polymerization Suspension Polymerization Emulsion Polymerization Monomer Solvent Initiator Polymer Monomer Water Suspending agent Initiator Polymer Monomer Water Emulsifier (Surfactant) Initiator Polymer Monomer is soluble in solvent. Initiator and Polymer may or may not be soluble in solvent. Monomer and Polymer are not soluble in water. Suspending agent is soluble in water. Initiator is soluble in monomer Initiator is soluble in water. Viscosity build-up. Removal of unreacted monomer is difficult. Heat of polymerization is taken up by a greater mass, thus easier temperature control. Viscosity increase is minimized (lower viscosity). Heat removal to the reactor wall is efficient (water better than an organic solvent because of higher heat conductivity and heat capacity) but temperature control is complicated (heat build-up in the droplets). Viscosity changes very little with conversion. Best temperature control. Viscosity changes very little with conversion. Monomer is only slightly soluble in water. 3. (2 pts) What will be the effect of the following chain transfer on the polymerization rate and the average degree of the polymerization if k3 ≈ k4 ≈ kp where kp is the rate constant for the propagation step of the radical chain reaction polymerization. k3 R′ + RM• Î RM + R′• k4 R′• + M Î R′M• & The overall rate will not change. Average degree of polymerization (i.e. ave. # of repeat units in the polymer chain) will decrease. 4. (3 pts) σ b c a ε List materials a, b and c from the most stiff to the least: a List materials a, b and c from the strongest to the weakest: > b b > c c > List materials a, b and c from the toughest to the most brittle: c > > a a > b 5. (3 pts) Plot qualitatively strain versus time (from t = 0 to t → ∞) for a viscoelastic material for the following loading. d σ c c>e>a=d>b a b e t ε d′ c′> e′> a′=d′> b′ c′ b′ e′ a′ t 6. (3 pts) Plot qualitatively strain versus time (from t = 0 to t → ∞) for a viscous material for the following loading. σ t ε t 7 (4 pts) Two samples (A and B) with narrow-molecular-weight distributions (Mn≈Mw) are prepared for a new polymer. Viscosity measurements in a solvent result in a solution viscosity of 1.43 poise at a concentration of 0.30 g/dl for Sample A and a solution viscosity of 1.63 poise at the same concentration for Sample B. Determine the molecular weight of sample B taking a=0.5, k ' = 0.5 , solvent viscosity = 1.1 poise and Molecular weight of Sample A = 8.50x104. 8. (4 pts) A polymer mixture ( M n =3.3×105, PDI=2.40) contains Polymer A (Mo=20, Mn=1.5×105, PDI=3.8), Polymer B (Mo=30 Mn=5.5×105, PDI=1.6) and Polymer C (Mo=50, Mn=7.5×105, PDI=1.0). Determine composition of the polymer mixture in weight fractions.