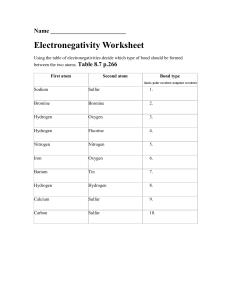
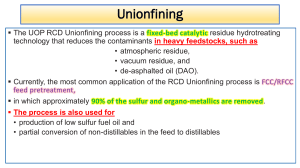
AP Chemistry Name: Exercise 2.7 Date: Structures and Naming I Per: DIRECTIONS: Complete the following in the space provided: 1. Observations of the reaction between nitrogen gas and hydrogen gas show us that 1 volume of nitrogen reacts with 3 volumes of hydrogen to make 2 volumes of gaseous product as shown below. Determine the formula of the product: 2. A sample of H2SO4 contains 2.02 g of hydrogen, 32.07 g of sulfur, and 64.00 g of oxygen. How many grams of sulfur and grams of oxygen are present in a second sample of H2SO4 containing 7.27 g of hydrogen. 3. For each of the following sets of elements, label each as either noble gases, halogens, alkali metals, alkaline earth metals, or transition metals: a. b. c. 4. Ti, Fe, Ag: Mg, Sr, Ba: Ne, Kr, Xe: d. e. F, Br, I: Li, K, Rb: Write the symbol of each atom using the AZX format: 10 electrons a. Revised: 2018-08-13 b. c. AP Chemistry Name: Exercise 2.7 Date: Structures and Naming I 5. Per: Complete the following chart: Symbol # of Protons # of Neutrons # of Electrons Net Charge 20 20 23 28 20 35 44 36 15 16 3- 26 33 3+ 85 125 86 13 14 10 76 54 238 92 U 53 26 6. 7. 2+ Fe 2 Name each of the following compounds: a. Rb2O: f. SeCl4: b. FeBr3: g. Sr3P2: c. S4N4: h. Co2S3: d. Ag2S: i. SO3: e. CuI2: j. AlI3: Write the formula for each of the following compounds: a. sulfur difluoride: f. manganese(IV) sulfide: b. lithium nitride: g. diphosphorus pentoxide: c. tin(II) chloride: h. cadmium selenide: d. carbon tetraiodide: i. chromium(VI) oxide: e. gallium arsenide: j. calcium iodide: Revised: 2018-08-13 2-