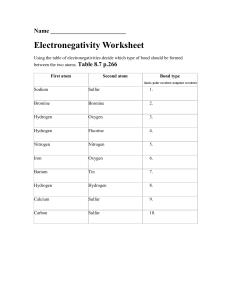
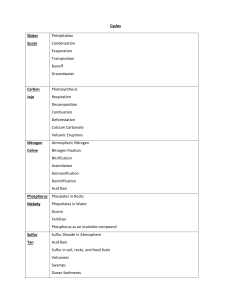
Unit 3 Skills Review 1. How many significant digits are in… a. 0.0012 _____ b. 92,000 _____ c. 72,010 _____ d. 120.00 _____ e. 0.001200 _____ f. 1.245 x 10-5 _______ 2, Give the correctly rounded answers… a. 12.345 + 23.1 + 8.9123 _____________ b. 345.00 x 2.122 _____________ c. 300 x 2.12 _____________ 3. Give the element represented by a. 1s22s22p63s23p64s23d9 b. [Ne]3s23p5 c. 6f3 4. Give the abbreviated EC electron configuration for… a. tellurium b. hafnium 5. In tantalum, how many protons? __________ electrons? ____________ nuetrons?_________ 6. How many valence electrons are in… a. phosphorus _________ b. argon __________ c. hydrogen _________ d. gold __________ 7. What is the valence charge of… a. selenium ______ 8. Give an example of… a. transition metal b. alkali metal b. helium ______ c. sulfate ________ d. sulfur ________ c. noble gas d. non-metal e. semiconductor 9. Draw the electron dot structure for… a. sulfur ________ b. helium __________ c. carbon __________ d. phosphorus ___________ 10. Give the chemical formula and word name for a compound made by combining… a. strontium and oxygen ________ _______________________________ b. strontium and chlorine ________ _______________________________ c. sulfur and tungsten ________ _______________________________ d. two bromine atoms ________ ________________________________ e. arsenic andosmium ________ ________________________________ f. magnesium and platinum ________ ________________________________ g. ammonium and chlorine ________ ________________________________ h. sodium and hydroxide ________ ________________________________ i. potassium and phosphate ________ ________________________________ j. acetate and magnesium ________ ________________________________ k. lithium and sulfate ________ ________________________________ l. hydrogen and cyanide ________ ________________________________ m. oxygen and hydrogen ________ ________________________________ n. oxygen and carbon ________ ________________________________