Water Treatment Methods: Environmental Engineering Presentation
advertisement

CEN 441 :
ENVIRONMENTAL ENGINEERING -II
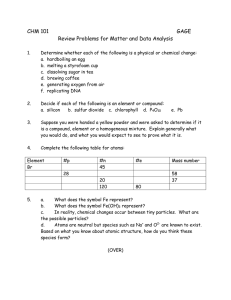
Chapter 01:
Water Treatment Methods
1
Dr AFM Kamal Chowdhury
Assistant Professor, Dept. of Civil Engineering, IUBAT
Office: 423, Cell: 01711- 479153
E-mail: afm.chowdhury@iubat.edu
CHAPTER COMPONENTS
Water Treatment Methods: Plain sedimentation,
sedimentation with coagulation, filtration, disinfection,
treatment of industrial water.
Waste Water: Estimation of waste water, wastewater
collection system, hydraulics of sewer, design, construction
and maintenance of sanitary sewer and storm sewer,
microbiology of waste water, primary and secondary
treatment of sewage.
Environmental Sanitation: Introduction, environmental
pollution, environment protection and management,
sanitation practices in Bangladesh.
Health and Hygiene: Diseases description, transmission
and control, hygiene education.
Pollution: Introduction to air pollution and noise
pollution.
Solid Waste Management: Solid waste collection,
transport, disposal and management.
2
WHY WE SHOULD
TREAT WATER
Natural water often contains impurities that are
harmful for human health
Common impurities include:
Impurities of mineral origin – iron, arsenic, lead, heavy
metals
Impurities of organic origin – vegetable dyes
Living impurities – bacteria, viruses, algae, protozoa, fungi
Radioactive impurities
These impurities may be present in suspension or
solution
3
WHY WE SHOULD
TREAT WATER
Some impurities might be detected by sight (turbidity,
colour), taste (salty, offensive) and smell (odour)
Detection of many pathogenic and poisonous
impurities require systematic laboratory tests
Scopes of water treatment:
Treatment for drinking water
Treatment of wastewater before disposing into waterbodies
4
BASIC REQUIREMENTS
OF DRINKING
WATER
Water should:
Be completely free of pathogenic micro-organisms that
can cause diseases
Contain no element or compound in concentrations
that can cause acute or long-term adverse effect on
human health
Be aesthetically acceptable – free of bad colour, taste
(e.g. salty), and smell
Not cause corrosion, scale formation, discoloration
Not have a temperature unacceptable to the
consumers
5
JUSTIFICATION FOR WASTEWATER
TREATMENT
Pollution from sewage is a primary environmental
health hazard (wastewater effluent).
The purpose of municipal wastewater treatment is to
limit pollution of the receiving watercourse.
The receiving watercourse may also be a source of
drinking water.
6
GOALS OF WASTEWATER TREATMENT:
Reduction of organic load of the wastewater effluent
to limit eutrophication (BOD, COD limits),
Reduction of microbiological contamination that may
transmit infectious disease.
7
ASSIGNMENT
Brief report on water quality parameters (Turbidity,
Total Dissolved Solids (TDS), Alkalinity, Hardness,
Nitrate, Total Coliform and Faecal Coliform, Iron,
Arsenic):
Definition/Causes/Source
Measurement procedure
In-Stream Acceptable Limits (WHO and Bangladesh
Standards)
Drinking Water Acceptable Limits (WHO and Bangladesh
Standards)
Impacts
8
WATER TREATMENT METHODS
9
COMMON WATER TREATMENT METHODS
Clarification - primarily a physical process (e.g. plain
sedimentation), but may be aided by addition of
chemicals (e.g. coagulation).
Filtration - also primarily physical, but chemicals may
aid the process.
Removes suspended and colloidal particles including color
producing substances.
Removes visible impurities.
Disinfection - typically a chemical process.
Reduces pathogenic microorganisms.
10
SOME SPECIFIC WATER TREATMENT METHODS
Aeration
Water softening
Iron removal
Activated carbon application
Fluoridation and defluoridation
Demineralization
Desalinization
11
COMMON APPLICATIONS
OF
WATER
TREATMENT METHODS
Surface water is turbid, colored and contaminated by
pathogenic micro-organisms and needs extensive
treatment such as sedimentation, coagulationsedimentation, filtration and disinfection.
Groundwater is usually hard (may require softening)
but free from pathogenic bacteria and can be supplied
for drinking purpose without treatment.
Some Tube-well water in Bangladesh may contain
iron, arsenic and hardness in excess of acceptable
levels, and may therefore require specific treatment.
12
TYPICAL SURFACE WATER TREATMENT
SYSTEM
13
PLAIN SEDIMENTATION
14
PLAIN SEDIMENTATION
Organic
or inorganic particles heavier than water (specific
gravity > 1) settle by retaining water in a tank or basin
These
particles are generally held in suspension in natural
water by turbulence or current
When
the current is retarded, particles heavier than water
tend to move downward by the force of gravity, accelerating
until the frictional resistance ('drag') of the water equals the
gravitational force acting upon the particles.
Thereafter
the particle travels with a constant vertical
velocity called the "terminal velocity' or 'settling velocity' of
the particle.
15
THE SETTLING VELOCITY OF THE PARTICLE
DEPENDS UPON
Horizontal
flow velocity of water
Shape and size of the particle
Specific gravity of the particle
Viscosity of water
Density of water
Temperature of water
16
17
SETTLING OF DIFFERENT TYPES OF
PARTICLES
Stoke's Law is valid for computation of settling velocity of
discrete particles
Discrete particles are those which do not change size,
shape and mass during settling and which do not influence
each other by being too close. Particle settling under this
conditions is called discrete settling
In case of closely packed particles, the water displaced by
the particles may cause additional friction and the settling
velocity is reduced. This is termed as hindered settling.
Hindered settling becomes noticeable when the
concentration of suspended solids is greater than 2,000
mg/1. This situation of high concentration of suspended
solids may happen in river water during high flooding and
18
heavy rainfall
SETTLING OF DIFFERENT TYPES OF
PARTICLES
Sometimes settling particles may adhere to each other
and grow in size and thus deviate from the settling
characteristics represented by Stoke's Law. This my
occur in settling of algae or freshly formed floc by the
process of flocculation with coagulant.
These particles/flocs tend to stick together and form
new bigger particles which settle at a faster rate. This
type of settling is called flocculent settling.
19
20
DESIGN OF SEDIMENTATION TANKS
A
rectangular sedimentation tank can be
subdivided into four different areas comprised of
an inlet, settling, outlet and sludge accumulation
zones
The inlet zone serves to provide even flow
distribution over the full cross section, the outlet
zone collects the clarified water over the full tank
width
Sludge is accumulated at the tank bottom where
it is stored and removed periodically
The settling zone shown in Figure is the most
important area where solid separation takes place
21
22
The efficiency of the settling tank in the removal of
suspended particles can be determined using limiting
settling velocity v0 of a particle which will just travel
the full depth (H) of the tank within the detention time
(T). Using the dimensions and notations used in Figure
the following equations can be written:
23
The
tank will remove all the particles having
settling velocity vs > vo and the particles with
settling velocity vs < vo will be removed in the
proportion vs : vo .
The
above analysis shows that the settling efficiency
depends on the ratio between the influent flow rate
Q and the surface area of the tank BL, which is
called the 'surface loading'.
Hence
the efficiency of the settling tank is
independent of the depth of the tank.
The
higher the surface area the greater is the
efficiency. Plate settlers and tube settlers have been
24
designed to provide a larger surface area and
achieve higher efficiency.
The
settling velocity of different fractions of
discrete particles can be computed by Stoke's Law
if the particle size distribution and specific gravity
of particle are determined by suitable methods.
The settling velocity of different fractions of
particles in water can be conveniently determined
by a settling column test of a representative
sample in the laboratory.
In the absence of column test data, the design
guideline given below may generally be followed
for good results
25
COAGULATION-FLOCCULATION
26
COAGULATION-FLOCCULATION PROCESS
A
chemical-aided clarification/sedimentation
process
Removes
colloids and very fine particles having
very low or no settling velocity, which cannot be
removed by plain-sedimentation
Coagulation
involves:
addition of a salt that produces positive ions in water
application of rapid mixing (hydraulic or mechanical)
destabilization of colloids
promotion of frequent contact among the particles
27
COAGULATION-FLOCCULATION PROCESS
Common Coagulants:
Aluminum sulphate
Ferric sulphate
Ferric chloride
Ferrous sulphate
– Al2(SO4)3.nH2O
– Fe2(SO4)3.9H2O
– Fe2Cl3.6H2O
– FeSO4
The Aluminium and Iron Salts react with natural
alkalinity of water and produce Aluminium and Iron
Hydroxides – Al(OH) 3 and Fe(OH) 3
The Al(OH) 3 and Fe(OH) are gelatinous (sticky) which
entrap the colloidal particles and form micro-flocs
28
COAGULATION-FLOCCULATION PROCESS
Flocculation – Sedimentation:
Gentle and continuous stirring for agglomeration of microflocs to produce larger flocs
The larger focs gain sufficient settling characteristics
and finally removed by sedimentation
29
EFFICIENCY OF COAGULATION
Eeach coagulant has optimum pH for best coagulation
Iron salt is very effective over a wider range of pH
Aluminium salt is most effective at a pH slightly higher than 7
30
EFFICIENCY OF COAGULATION
If required alkalinity is not naturally present in water,
alkalinity is added as Ca(OH)3 or Na2CO3
Mixing should be rapid for immediate dispersal of
coagulant throughout the raw water
Two types of mixing:
• Hydraulic rapid mixing (e.g. by producing turbulent
condition in baffled channels, or by feeding
coagulants at the suction side of the pump)
• Mechanical rapid mixing (e.g. paddles, propellers,
turbines etc. which require continuous power
supply)
31
32
33
PROBLEM ON COAGULATION-FLOCCULATION
Example 4.4 and Example 4.5 from Peavy’s Book
34
FILTRATION
35
FILTRATION
Water is allowed to pass through a bed of filtering media
usually sand and gravel.
Common filtration methods:
Mechanical staining
Sedimentation and adsorption
Microbial action
Electrostatic attraction
36
MECHANICAL STRAINING
Large particles that cannot pass through the thin
openings between the sand grains are retained in the
top layer of the filter media.
Accumulated material in the top layer of the bed
increases the straining efficiency but decreases the
downward flow of water.
Cannot remove bacteria and colloidal matter.
37
SEDIMENTATION AND ADSORPTION
Pores
in the sand bed act as a minute
sedimentation basin.
Curved
flow paths around grains bring the
fine particles and bacteria in contact with
sand surfaces.
Sticky
gelatinous coatings are formed on the
sand grains which retain the colloids, small
particles and bacteria.
38
MICROBIAL ACTION
A part of the organic material present in raw water is
transformed into cell materials for microbial growth.
A coating of micro-organisms is formed around the sand
grains which retain the organic matters and bacteria.
39
ELECTROSTATIC ATTRACTION
Sand
particles with negative surface charges
cannot attract negatively charged bacteria and
colloids
With
continuous adsorption of positively charged
particles and ions, the negatively charged sand
surface turned into positively charged surface.
Overall
charge of filter bed becomes positive
which attract and retain the negatively charged
bacteria and colloids.
40
ROUGHING FILTER
It is used for pretreatment of very turbid water
Consists of different sizes of gravels or stone chips
Gravel layers of different sizes are installed with gravel
size decreasing in the direction of flow
Three common types:
Down-flow roughing filters
Up-flow roughing filters
Horizontal-flow roughing fitters
41
42
43
EFFICIENCY OF ROUGHING FILTRATION
Suspended
Turbidity
solids removal of up to 95%
removal between 50 and 90%
Colour removal between 20 to 50%
Faecal
coliform reduction between 0.65 and 2.5
log units
Around 50% removal of iron and manganese
from groundwater
44
SLOW SAND FILTRATION (SSF)
Water is allowed to pass through a bed of fine sand
Retains most of the impurities including fine organic
and inorganic solid matters, dissolved (i.e. oxidized)
organic compounds and micro-organisms
Suitable for development of a surface water-based
water supply system in developing countries
45
CHARACTERISTICS OF SSF
Rate
of filtration is low, 0.1-0.3 m3 per m2 per hr
Very
high removal of turbidity and
colour (80-85%) and bacteria (95-99.9 %)
Cleaning
of filter bed by scraping and removal
of a top layer of sand
Not
suitable for water having turbidity greater
than 30 NTU
Low-cost
of operation and maintenance
46
DESIGN
An
open tank of around 2m height containing:
a sand bed of approximately 0.5-0.7 m thickness
around 1 m depth of water and
0.1 m freeboard
underdrain system of gravel with 0.3 to 0.5 m height
to collect clean water
Water
flows by gravity through the filter bed
Filtration
rate should be between 0.1 to 0.2 m/hr
Sand bed should have an effective size, d10
between 0.1 and 0.3 mm and a uniformity
coefficient d60/d10 below 3
47
48
COMBINED ROUGHING AND SLOW SAND
FILTERS
Slow
sand filters do not work when the turbidity
exceeds 30 NTU.
SSF
in Bangladesh require frequent washing for
high turbidities.
Pre-treatment by roughing filters can reduce
the load on SSFs
Filter
can operate for a longer period of time
between cleaning
49
50
RAPID SAND FILTER
High
filtration rate of 5-15 m3/m2/hr
High
filtration rate is achieved by using coarse
sand with an effective size of 0.4-1.2 mm
Filter
bed: a coarse sand layer of 1m laid on top of
a gravel layer of 0.5m
Can
be both gravity type and pressure type
Cleaning
by backwashing - water is directed in the
reverse direction at a high rate of flow
High
removal of turbidity and colour (80-85%) and
bacteria (85-95%)
Pre-treatment
Higher
is required
cost of operation and maintenance
51
RAPID SAND FILTER
Filter
size is determined by the required
capacity of the plant
Number
of unit of the plant is determined by
the empirical equation:
N = 0.04 √Q
where, N = Number of Units
Q = Plant Capacity in m3/day
Solve
Example 2 of Chapter 18 from Ahmed
and Rahman
52
DISINFECTION
53
DISINFECTION
Processes
of destruction or at least
complete inactivation of pathogens present
in water.
54
PHYSICAL DISINFECTION
Boiling
Ultraviolet Rays
Sunlight
55
CHEMICAL DISINFECTION
Quick and effective in killing pathogenic microorganisms present in water
Rapidly soluble in water in concentrations required for
disinfection and capable of providing a residual for
subsequent protection of water
Not imparting taste, odour, colour or turbidity to water
Not toxic to human and animal life
Easy to detect and measure in water
Easy to handle, transport, apply and control
Readily available at moderate cost
56
57
IMPORTANT TERMINOLOGY
Combined Available Chlorine
Free Available Chlorine
Chlorine Demand
Break point chlorination
58