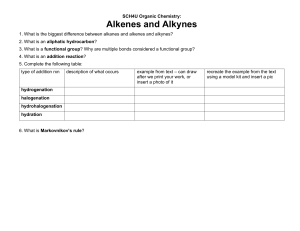
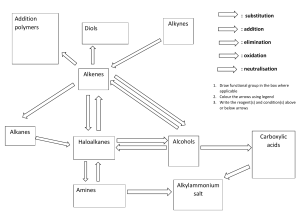
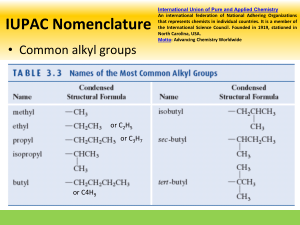
1. Organic chemistry is the chemistry of carbon. 2. The name “organic” reflect the fact that organic molecules are derived from living or nonliving organisms? 3. 3 Forms of Pure Carbon - Graphite 4. Diamond 5. Buckminsterfullerene - “Bucky Balls” 6. Hydrocarbons – elements? Hydrogen 7. Carbon 8. Alkanes 9. Alkenes 10. Alkynes 11. Aromatics 12. Propane – Alkane 13. Propene - Alkene 14. Propyne - Alkyne 15. Benzene – Aromatic Compounds 16. Methane – CH4 17. Ethane – CH3CH3 18. Alkanes in which the carbons are connected in a straight chain are called normal alkanes. 19. Alkanes interact with each other only through London dispersion forces. 20. Alkanes have relatively low or high boiling and melting points. 21. Use of diamond? 22. Alkanes – Saturated 23. Alkenes – Unsaturated 24. Alkynes – Unsaturated 25. Aromatic Compounds – Unsaturated 26. Alkanes, along with the other hydrocarbons, are non-polar or polar? 27. Melamine 28. When two or more molecules share the same molecular formula, but have different atomic connections, they are called constitutional isomers. 29. Conformations – TRUE OR FALSE - Carbon-carbon single bonds are free to rotate. 30. Conformations - TRUE OR FALSE - They all share the same structural formula.