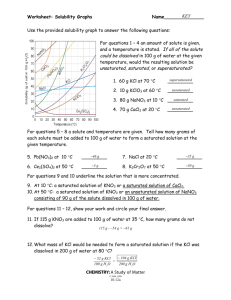
SCIENCE 7 SATURATED AND UNSATURATED SOLUTION WHAT IS A SOLUTION? Solution is a homogenous mixture of two components: solute and solvent. WHAT IS THE DIFFERENCE BETWEEN SOLUTE AND SOLVENT? The solute is the component that is present in the minority and the solvent is the component that is present in the majority. A solution in which solutes dissolve is referred to as an unsaturated solution. A solution is made up of two types of particles: solutes and solvents. Water is commonly used as a solvent (which is one of the reasons why water is also called the universal solvent). EXAMPLE: A solution of sugar in water A solution of salt in water A solution of air in water A solution of oxygen in nitrogen WHAT ARE THE TYPES OF SOLUTIONS? SATURATED UNSATURATED SUPERSATURATED SATURATED A saturated solution is a chemical solution that contains the largest quantity of solute contained in the solvent at the given temperature. The solute cannot be dissolved anymore in a saturated solution at that temperature. SATURATED The saturation point of any liquid is determined by the type of the material and the temperature. A saturated solution is one in which the quantity of dissolved solute equals the saturation point of the solvent. A solvent can dissolve some particular types of solutes in it. SATURATED SOLUTION EXAMPLE •Soil is a saturated mixture consisting of nitrogen. On attaining the saturation point, the excess nitrogen is emitted out into the air in the form of gas. •Beverages, such as cold drinks are saturated solutions of dissolved CO2 in water. •Protein drinks which is a saturated solution of protein powder in milk or any other solvent UNSATURATED •The solution in which more solute can be added at a given temperature is called unsaturated solution. • Unsaturated solutions have the ability to dissolve additional solute until they achieve saturation. Solutes will no longer dissolve in the solvent after reaching the saturation threshold, resulting in unsaturated solutions. UNSATURATED As a result, all solutions are considered to be largely unsaturated in nature before being transformed into saturated solutions by adding solute to them. The amount of solute that is contained in lesser amounts than the maximum value, that is before the solution reaches the saturation level is called an unsaturated solution. An unsaturated solution is basically a chemical solution that has a solute concentration lesser than its corresponding equilibrium solubility. UNSATURATED SOLUTION EXAMPLES •Salt or sugar dissolved in the water below the saturation point. •Air or mist. •Iced coffee. •Vinegar is the acetic acid solution in water Difference between Saturated and Unsaturated Solution The key differences between Saturated and Unsaturated solutions are as follows: How to find whether the given Solution is Saturated or Unsaturated? Step 1: Add a small amount of solute to the solution. Step 2: Stir the solution thoroughly. Step 3: If the solute dissolves, the solution is unsaturated. Step 4: If the solute does not dissolve, the solution is saturated