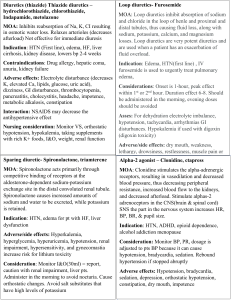
EJCCM 25 9 June 2014 The Egyptian Journal of Critical Care Medicine (2014) xxx, xxx–xxx No. of Pages 6 1 The Egyptian College of Critical Care Physicians The Egyptian Journal of Critical Care Medicine http://ees.elsevier.com/ejccm www.sciencedirect.com ORIGINAL ARTICLE 2 6 Assessment of hypotension during dialysis as a manifestation of myocardial ischemia Q1 in patients with chronic renal failure 7 Q2 4 5 a 8 9 10 Randa Aly Soliman Q3 b a,* , Mohamed Fawzy a, Hussein Kandil b, Alia Abd el Fattah a The Critical Care Department, Cairo university, Egypt The Critical Department in the National Liver Institute, Egypt Received 4 September 2013; revised 3 May 2014; accepted 15 May 2014 11 13 14 KEYWORDS 15 Intradialytic hypotension (IDH); With chronic renal failure (CRF); Myocardial perfusion; Imaging (MPI) 16 17 18 19 20 Abstract Introduction: Intradialytic hypotension (IDH) remains to be a major complication of hemodialysis occurring in nearly 25% of dialysis sessions. It is a significant independent factor affecting mortality in hemodialysis patients. Autonomic nervous system dysfunction, blood sequestration in the setting of hypovolemia, cardiovascular diseases and increased plasma level of end products of nitric oxide metabolism are possible causes. In this controlled prospective study we examined patients with chronic renal failure and intradialytic hypotension to evaluate the relationship between this hypotension and myocardial ischemia after controlling other possible causes. Materials and methods: Thirty patients with chronic renal failure who are on regular dialysis were enrolled. Before dialysis, patients were subjected to history taking and clinical examination. Echocardiography and several lab tests were done. Glomerular filtration rate (GFR) was calculated using Cockcroft’s and Gault formula. Autonomic dysfunction was also assessed. The dialysis session was standardized in all patients. Intradialytic blood pressure was monitored and hypotension was classified as mild (SBP > 100 mmHg), moderate (SBP 80–100) or severe (SBP < 80). After dialysis, myocardial ischemia was assessed using stress myocardial perfusion imaging (MPI) (Pharmacologic stress testing using Dipyridamole) and is further classified as mild, moderate or severe ischemia. Patients with sepsis, hemoglobin level less than 9 g/dL, diabetes mellitus, low cardiac output, coronary artery disease, significant valvular lesion or body weight below the dry weight of the patient were excluded from the study. Bronchial asthma, emphysema and severe COPD are contraindications to Dipyridamole and thus were also excluded from the study. Results: Twenty patients had no or mild intradialytic hypotension whereas ten patients had * Corresponding author. Address: 20 Abou Hazem st., Madkour, Haram, Giza, Egypt. Tel.: +20 1222402018. E-mail address: randaalysoliman@hotmail.com (R.A. Soliman). Peer review under responsibility of The Egyptian College of Critical Care Physicians. Production and hosting by Elsevier 2090-7303 2014 Production and hosting by Elsevier B.V. on behalf of The Egyptian College of Critical Care Physicians. http://dx.doi.org/10.1016/j.ejccm.2014.05.001 Please cite this article in press as: Soliman RA et al. Assessment of hypotension during dialysis as a manifestation of myocardial ischemia in Q1 patients with chronic renal failure, Egypt J Crit Care Med (2014), http://dx.doi.org/10.1016/j.ejccm.2014.05.001 EJCCM 25 9 June 2014 No. of Pages 6 2 R.A. Soliman et al. moderate or severe hypotension. Among the first group, only two patients (10%) were found to have myocardial ischemia, while in the latter group, seven patients (70%) had myocardial ischemia that is mostly moderate (p = 0.002). Stress induced LV dysfunction was also significantly prevalent in patients with moderate or severe intradialytic hypotension as opposed to the other group (p = 0.002) LVED. Conclusions: Patients with CKD and regular hemodialysis who experience moderate or severe intradialytic hypotension have significantly higher prevalence of myocardial ischemia and stress induced myocardial dysfunction, than those who experience no or mild intradialytic hypotension. 21 22 23 24 25 26 27 28 32 29 30 2014 Production and hosting by Elsevier B.V. on behalf of The Egyptian College of Critical Care Physicians. 31 33 1. Introduction 34 67 Intradialytic hypotension (IDH) remains to be a major complication of hemodialysis. It occurs in nearly 25% of dialysis sessions [1] and often requires aggressive resuscitative measures and sometimes premature termination of hemodialysis. It is also a significant independent factor affecting mortality in hemodialysis patients [2]. Despite the advances of machines with ultrafiltration control devices, modifying dialysate composition, temperature control, correction of nutritional deficiencies and treatment of anemia with erythropoietin therapy, many patients still have episodes of intradialytic hypotension. Among other factors, the major pathophysiology of these episodes is the removal of large volume of blood water and solutes over a short period of time, overwhelming normal compensatory mechanisms, which include plasma refilling and reduction of venous capacity (due to reduction of pressure transmission to veins). In some patients, a seemingly paradoxical and inappropriate reduction of sympathetic tone may occur, causing reduction of arteriolar resistance, decreased transmission of pressure to veins with corresponding increase in venous capacity. Increased sequestration of blood in veins under conditions of hypovolemia reduces cardiac filling, cardiac output and ultimately blood pressure. Hypotensive episodes during hemodialysis in patients with end stage renal disease in the absence of inadequate maintenance of the plasma volume, pre-existence of cardiovascular disease, or autonomic nervous system dysfunction are accompanied by increased plasma concentrations of the end-products of nitric oxide metabolism (above the expected levels, based on the reduction of urea [3]). In this controlled prospective study, patients with chronic renal failure and intradialytic hypotension episodes were thoroughly investigated, to evaluate the relationship between hypotension and myocardial ischemia after controlling other possible causes. 68 2. Patients and methods 69 This prospective study was conducted in King Fahd Hemodialysis unit in Kasr Al-Aini hospital, Hemodialysis Unit in AlZahraa hospital and Critical Care Medicine Department in Kasr Al-Aini hospital over the time period from February 2010 to June 2011. All patients included in the study provided informed written consent. The study includes 30 patients with chronic renal failure who receive regular hemodialysis sessions. Twenty patients developed hypotension during hemodialysis session, and the remaining 10 patients did not develop hypo- 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 70 71 72 73 74 75 76 77 Table 1 Baseline characteristics of the study group (n = 30). Parameter Value [N (%) or mean ± SD] Age (years) Weight (Kg) Male 44 ± 13 66 ± 10 12 (40) Clinical Smokers Hypertension History of chest pain Family history of IHD Autonomic neuropathy AV fistula Duration of hemodialysis (years) 7 (23) 12 (40) 6 (20) 9 (30) 9 (30) 23 (77) 3.7 ± 2.1 ECG and ECHO Arrhythmia in ECG LVH in ECG RWMA LVEDD (mm) LVESD (mm) EF (%) 4 (13) 6 (20) 5 (17) 49.7 ± 5.4 32.4 ± 4 62.3 ± 7.6 Grades of Albuminuria* (+) (++) (+++) Urine specific gravity* 6 (20) 7 (23) 2 (7) 1009 ± 2.1 Labs Hb (g/dL) Hct (%) TLC (103 cells/cmm) FBS (mg/dL) PPBS (mg/dL) HB A1C (mg/dL) Serum Triglycerides (mg/dL) Serum Cholesterol (mg/dL) Serum Urea (mg/dL) Serum Creatinine (mg/dL) GFR (ml/min/1.73 m2) Serum Sodium (mEq/L) Serum Potassium (mEq/L) 10.5 ± 0.73 32.6 ± 2.9 6.1 ± 2.1 70.2 ± 11.2 123.5 ± 15.2 5 ± 0.59 109.7 ± 43 151 ± 60 129.8 ± 29.7 7.9 ± 2.1 11 ± 4.2 138 ± 5.8 5.2 ± 0.63 IHD, ischemic heart disease; LVH, left ventricular hypertrophy; RWMA, regional wall motion abnormalities; LVEDD, left ventricle end-diastolic diameter; LVESD, left ventricle end-systolic diameter; EF, ejection fraction Hb, hemoglobin; Hct, hematocrit; TLC, total leukocytic count; FBS, fasting blood sugar; PPBS, post prandial blood sugar; HB A1C, hemoglobin A1C; GFR, glomerular filtration rate. * Done only for the 15 patients who were not anuric. Please cite this article in press as: Soliman RA et al. Assessment of hypotension during dialysis as a manifestation of myocardial ischemia in Q1 patients with chronic renal failure, Egypt J Crit Care Med (2014), http://dx.doi.org/10.1016/j.ejccm.2014.05.001 EJCCM 25 9 June 2014 No. of Pages 6 Q1 Assessment of hypotension during dialysis as a manifestation of myocardial ischemia 3 Figure 1 Myocardial ischemia diagnosed by MPI in patients with moderate or severe intradialytic hypotension, in comparison to those with no or mild hypotension (p = 0.002). 87 tension during the sessions. Myocardial ischemia was assessed in all patients using stress myocardial perfusion imaging (MPI) (Pharmacologic stress testing using Dipyridamole). Patients with sepsis, hemoglobin level less than 9 g/dL, diabetes mellitus, low cardiac output, acute coronary syndrome, significant valvular lesion or body weight below the dry weight of the patient were excluded from the study. Bronchial Asthma, emphysema and severe COPD are contraindications to Dipyridamole and thus were also excluded from the study. 88 2.1. Before dialysis 89 All eligible patients were subjected to full history taking and clinical examination. Serum Urea and Creatinine were measured. Glomerular filtration rate (GFR) was calculated using Cockcroft’s and Gault equation. Autonomic dysfunction was assessed using at least 2 of the following tests; blood pressure (BP) response to standing, BP response to sustained handgrip, Heart Rate (HR) response to standing, HR response to deep breathing and HR response to valsalva. Positive result of any test indicates autonomic dysfunction [4]. 78 79 80 81 82 83 84 85 86 90 91 92 93 94 95 96 97 98 2.2. During dialysis 99 Dialysis was done via AV fistula (23 patients) or dialysis catheter (7 patients), using Fresenius 4008B, Nipro machine with ultrafiltration volume control and polysulfone, Fresenius F6 filters. Temperature of dialysate was kept at 36 C. Blood pump was kept between 250 and 350 ml/min except during the hypotensive episodes during which the blood pump was decreased to only 200 ml/min and not less to insure adequate dialysis session. Dialysate flow was 500 ml/min. All dialysis sessions lasted around 4 h. BP is recorded using standard sphygmomanometer every 30 min in supine position. Each time the mean of 3 measurements is recorded. Intradialytic hypotension is defined as a symptomatic decrease of more than 30 mmHg in systolic blood pressure or as an absolute systolic blood pressure under 90 mmHg [5]. Hypotension is further classified as mild (Systolic Blood Pressure (SBP) > 100 mmHg), moderate (SBP 80–100 mmHg) and Severe (SBP < 80 mmHg) [6]. 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 Patients who required vasopressors were unstable and accordingly were excluded from this study. 116 2.3. After dialysis 118 Within 2–5 h after dialysis, patients had both trans-thoracic echocardiography and MPI. The echo was done using an ATL machine HDI 5000 with the patient lying in the left lateral decubitus using a 3.5 MHZ probe. MPI was done at the nuclear laboratory of the critical care medicine department, Kasr Al-Aini hospital, Cairo University utilizing the ‘‘freeze imaging protocol’’. The set of SPECT images was acquired using a triple head Siemens gamma camera with high resolution collimators (model Symbia E). Pharmacological stress testing using Dipyridamole was done as most patients with CKD could not achieve target HR during treadmill stress testing due to marked physical limitations. Patients were instructed to fast for at least 6–8 h, stop theophylline medications for at least 24 h and not to have any caffeinated drinks or beverages for at least 24 h prior to the study. Dipyridamole 0.56 mg/kg was diluted with 40 cc normal saline and infused over 4 min. 2 min later, 20–25 mCi Tc-99 m Sestamibi were injected intravenously through a wide bore cannula followed by saline flush. Patients were monitored for at least 5 min or till vital signs returned to baseline. Ambulant patients were asked to walk for 4 min after Dipyridamole infusion. Twenty projections were acquired (30 s for each frame) at 120 degree arc and total acquisition time of 12 min. SPECT images were processed using the back-projection technique to get trans-axial images then short axis, vertical long axis and horizontal long axis cuts. The twenty-segment scoring system was applied to estimate the Myocardium At Risk (MAR), and the severity of perfusion defect was assessed for each segment using a ‘‘0–4’’ scoring system with ‘‘0’’ indicating normal perfusion and ‘‘4’’ indicating no photon activity. The sum of these scores is the Summed Stress Score (SSS). Seventy two hours later, patients were re-injected with 20– 30 mCi Tc-99 m Sestamibi intravenously to acquire the second set of SPECT images at rest, and to estimate the left ventricular ejection fraction (EF) utilizing the Gated SPECT technique. The severity of perfusion defects of MAR in this set of SPECT images is assessed similarly and the sum of these scores is the Summed Rest Score (SRS). 119 Please cite this article in press as: Soliman RA et al. Assessment of hypotension during dialysis as a manifestation of myocardial ischemia in Q1 patients with chronic renal failure, Egypt J Crit Care Med (2014), http://dx.doi.org/10.1016/j.ejccm.2014.05.001 117 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 EJCCM 25 9 June 2014 No. of Pages 6 4 R.A. Soliman et al. Table 2 Comparison of demographic, clinical, laboratory and MPI data between the 2 study groups. Parameter Group A (n = 20) Group B (n = 10) Age (years) Weight (Kg) Male 43.5 (33–54) 65.5 (56–73) 9 (45) 47.5 (28–58) 67 (58–77) 3 (30) 0.63 0.81 0.69 Clinical Smokers Hypertension Family history of IHD Autonomic neuropathy AV fistula Duration of hemodialysis (years) 5 (25) 7 (35) 5 (25) 4 (20) 16 (80) 4 (3–6) 2 (20) 5 (50) 4 (20) 5 (50) 7 (70) 2.5 (1–4) 1.0 0.69 0.43 0.115 0.66 0.1 ECG and ECHO Arrhythmia in ECG LVH in ECG RWMA LVEDD (mm) LVESD (mm) EF (%) 1 (5) 3 (15) 2 (10) 52 (46.5–55) 32 (30–35) 63 (56–70) 3 (30) 3 (30) 3 (30) 44.5 (44–51) 31.5 (28–34) 59.5 (56–62) 0.095 0.37 0.30 0.046 0.63 0.19 Grades of Albuminuria* (+) (++) (+++) Urine specific gravity n=8 5/8 (62.5) 3/8 (37.5) 0/8 1010 (1008–1010) n=7 1/7 (14.3) 4/7 (57.1) 2/7 (28.6) 1009 (1005–1010) 0.12 Labs Hb (g/dL) Hct (%) TLC (103 cells/cmm) FBS (mg/dL) PPBS (mg/dL) HB A1C (mg/dL) Serum Triglycerides (mg/dL) Serum Cholesterol (mg/dL) Serum Urea (mg/dL) Serum Creatinine (mg/dL) GFR (ml/min/1.73 m2) Serum Sodium (mEq/L) Serum Potassium (mEq/L) 10.4 (9.8–10.9) 31.9 (30.3–35) 5.4 (4.8–7.6) 71 (60–80) 123 (116–131) 4.9 (4.6–5.5) 90.5 (80–117) 135 (113–165) 138 (101–151) 8.4 (6.3–9.7) 10.2 (7.9–12.4) 138.5 (133–144) 5.2 (4.7–5.5) 10.5 (10.3–11.3) 33 (32.3–37) 5.2 (4–8) 69 (62–76) 119 (108–134) 4.8 (4.3–5.5) 97 (75–170) 117 (109–247) 121 (106–144) 7.7 (6.5–9) 9.5 (8.9–12.6) 138.5 (133–142) 5.3 (5–6.1) 0.15 0.098 0.74 0.88 0.58 0.42 0.75 0.55 0.4 0.63 0.55 1.0 0.094 MPI Ischemia (total count) Mild ischemia Moderate ischemia Scar Stress induced LV dysfunction 2 (10) 1/1 (50) 1/1 (50) 0 2 (10) 7 (70) 1/7 (14.3) 6/7 (85.7) 2 (20) 7 (70) 0.002 0.417 0.092 0.103 0.002 Data are displayed as n (%) or median (inter-quartile range). IHD, ischemic heart disease; LVH, left ventricular hypertrophy; RWMA, regional wall motion abnormalities; LVEDD, left ventricle enddiastolic diameter; LVESD, left ventricle end-systolic diameter; EF, ejection fraction; Hb, hemoglobin; Hct, hematocrit; TLC, total leukocytic count; FBS, fasting blood sugar; PPBS, post prandial blood sugar; HB A1C, hemoglobin A1C; GFR, glomerular filtration rate. * Done only for the 15 patients who were not anuric. 157 158 159 160 The difference between SSS and SRS is the Summed Difference Score (SDS). It is classified as follows; 0–4 indicates no ischemia, 5–8 mild ischemia, 9–12 moderate ischemia and more than 12 is severe ischemia. 161 2.4. Statistical methods 162 Statistical analysis was done using Statistical Package for Social Sciences (SPSS) software, release 16.0.0 for Windows (SPSS Inc., Chicago, Illinois). 163 164 Categorical variables are described as frequency (n) and percentage (%). Quantitative variables are described as mean ± standard deviation (SD) whenever parametric. Nonparametric quantitative variables are described as median and interquartile range (IQR). Bivariate analysis of categorical variables was done using Chi-square test with Yates Continuity correction for 2 · 2 tables. Whenever cell frequency is <5, Fisher’s Exact test is used. Comparison of two groups of quantitative variables was done using Independent-Samples Student’s t test for parametric data, and Mann–Whitney test for non-parametric data. Please cite this article in press as: Soliman RA et al. Assessment of hypotension during dialysis as a manifestation of myocardial ischemia in Q1 patients with chronic renal failure, Egypt J Crit Care Med (2014), http://dx.doi.org/10.1016/j.ejccm.2014.05.001 165 166 167 168 169 170 171 172 173 174 175 EJCCM 25 9 June 2014 No. of Pages 6 Q1 Assessment of hypotension during dialysis as a manifestation of myocardial ischemia 5 Figure 2 Stress induced LV dysfunction diagnosed by MPI in patients with moderate or severe intradialytic hypotension in comparison to those with no or mild hypotension (p = 0.002). Figure 3 Left ventricular end-diastolic diameter in patients with moderate or severe intradialytic hypotension (44.5 mm [44–51]) in comparison to those with no or mild intradialytic hypotension (52 mm [46.5–55]) (p = 0.046). In all cases, the 2-sided significance was always taken as p value. p value <0.05 is considered statistically significant. 52 mm, IQR 46.5–55) than in Group B (44.5 mm, 44–50.75) (p = 0.046) (Fig. 3). 191 177 178 3. Results 4. Discussion 193 179 Thirty patients were included in this study. Their baseline characteristics are listed in Table 1. We found that 70% (7/10) of patients who had moderate or severe hypotension (Group B) had myocardial ischemia on MPI, in comparison to 10% (2/20) of patients who experienced no or mild intradialytic hypotension (Group A); a difference that is statistically significant (p = 0.002) (Fig. 1). Several other variables were compared across both study groups (Table 2). It is to be noted that patients of Group B were more likely to have stress induced LV dysfunction on MPI (7/10, 70%) than patients of Group A (2/20, 10%) (p = 0.002) (Fig. 2). LVED was wider in Group A (Median Intradialytic hypotension (IDH) is a major complication of hemodialysis. Two to four liters of fluid needs to be removed during a regular session, equivalent to 40–80% of the blood volume. It is therefore not surprising that hypotension occurs so often. Although many factors – patient or treatment related – play a role, a reduction of blood volume is crucial in its pathogenesis [1]. Hypotension is one of the clinical presentations of CVD in patients with CKD [7]. There are a number of possible explanations for the independent association of reduced GFR and CVD outcomes. First, a reduced GFR may be associated with an increased level of nontraditional CVD risk factors that fre- 194 176 180 181 182 183 184 185 186 187 188 189 190 Please cite this article in press as: Soliman RA et al. Assessment of hypotension during dialysis as a manifestation of myocardial ischemia in Q1 patients with chronic renal failure, Egypt J Crit Care Med (2014), http://dx.doi.org/10.1016/j.ejccm.2014.05.001 192 195 196 197 198 199 200 201 202 203 204 205 EJCCM 25 9 June 2014 No. of Pages 6 6 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266 quently are not assessed in many studies. Second, reduced GFR may be a marker of undiagnosed vascular disease or alternatively a marker of the severity of diagnosed vascular disease, especially in high or highest risk populations. Third, reduced GFR may have had more severe hypertension or dyslipidemia and therefore have suffered more vascular damage secondary to hypertension or dyslipidemia. Fourth, recent studies have suggested that subjects with reduced GFR are less likely to receive medications or therapies such as angiotensin converting enzyme inhibitors, ß-blockers, aspirin, platelet inhibitors, thrombolytics, or percutaneous intervention than patients with preserved GFR. Perhaps as important was the fact that in the same studies, patients with reduced GFR who did receive the above interventions obtained similar benefit as patients with preserved GFR. Finally, decreased GFR itself may be a risk factor for progression of ventricular remodeling and cardiac dysfunction [7]. Different studies stated that, during hemodialysis, patients are particularly susceptible to myocardial ischemia for number of reasons including: LV hypertrophy [8], intradialytic hypotension and instability [9], high prevalence of decreased coronary flow reserve even in the absence of coronary vessel stenosis [10,11]. Hakeem et al. [12] reported that in patients with CKD 40% of perfusion scans were abnormal (SSS P 4) with 20% mild defects and 20% moderated to severe defects. Q4 In line with Hakeem’s result, we found that patients who experience moderate or severe intradialytic hypotension have significantly higher prevalence of myocardial ischemia (70%, 7/10), in comparison to those who have no or mild intradialytic hypotension (10%, 2/20) (p = 0.002). Paoletti et al. [13] stated that there is growing evidence that patients with CKD have unrecognized LV dysfunction, both systolic and diastolic. They also pointed at the importance of LV dysfunction (LV ejection fraction <40%) as a predictor of cardiac death in patients with CKD. We similarly found that CKD patients who develop moderate or severe intradialytic hypotension, have significantly higher prevalence of stress induced LV dysfunction (70%, 7/ 10) than those who have no or mild hypotensive episodes (10%, 2/20) (p = 0.002). Deterioration of renal function in CKD may lead to dyslipidemia or accumulation of uremic toxins, which can stimulate oxidative stress and inflammation, which in turn contributes to endothelial dysfunction and progression of atherosclerosis [14]. Renal failure causes changes in plasma components and endothelial structure and function that favor vascular injury, which may play a role as a trigger for inflammatory response. Dyslipidemia associated with CKD contributes to the inflammatory response in renal failure [15]. However, in our study we found that levels of serum cholesterol and triglycerides were not significantly different between the two study groups, a finding probably attributed to the small number of the study group and the relatively low economic standard of those patients. Patients with CKD also have a high prevalence of arteriosclerosis and remodeling of large arteries. Remodeling may be due to either pressure overload – which is distinguished by wall hypertrophy and an increased wall-to-lumen ratio – or flow overload, which is characterized by a proportional increase in arterial diameter and wall thickness [16]. R.A. Soliman et al. In conclusion, we found that patients with CKD and regular hemodialysis who experience moderate or severe intradialytic hypotension have significantly higher prevalence of myocardial ischemia and stress induced myocardial dysfunction, than those who experience no or mild intradialytic hypotension. 267 References 273 [1] Ligtenberg G, Blankestijn PJ, Boomsma F, Koomans HA. No change in automatic function tests during uncomplicated haemodialysis. Nephrol Dial Transplant 1996;11(4):651–6. [2] Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 2004;66(3):1212–20. [3] Madore F, Prud’homme L, Austin JS, Blaise G, Francoeur M, Leveille M, et al. Impact of nitric oxide on blood pressure in hemodialysis patients. Am J Kidney Dis 1997;30(5):665–71. [4] Ewing DJ, Campbell IW, Murray A, Neilson JM, Clarke BF. Immediate heart-rate response to standing: simple test for autonomic neuropathy in diabetes. Br Med J 1978;1(6106):145–7. [5] Straver B, Hypotension during hemodialysis. Noninvasivce monitoring of hemodynamic variables. 2005, The Dutch Kidney Foundation (Nierstichting Nederland, project C 94–1391). [6] Hanss R, Bein B, Ledowski T, Lehmkuhl M, Ohnesorge H, Scherkl W, et al. Heart rate variability predicts severe hypotension after spinal anesthesia for elective cesarean delivery. Anesthesiology 2005;102(6):1086–93. [7] Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003;42(5):1050–65. [8] Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int 1989;36(2):286–90. [9] Bos WJ, Bruin S, van Olden RW, Keur I, Wesseling KH, Westerhof N, et al. Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am J Kidney Dis 2000;35(5):819–26. [10] Ragosta M, Samady H, Isaacs RB, Gimple LW, Sarembock IJ, Powers ER. Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. Am Heart J 2004;147(6):1017–23. [11] Tok D, Gullu H, Erdogan D, Topcu S, Ciftci O, Yildirim I, et al. Impaired coronary flow reserve in hemodialysis patients: a transthoracic Doppler echocardiographic study. Nephron Clin Pract 2005;101(4):c200–6. [12] Hakeem A, Bhatti S, Dillie KS, Cook JR, Samad Z, Roth-Cline MD, et al. Predictive value of myocardial perfusion single-photon emission computed tomography and the impact of renal function on cardiac death. Circulation 2008;118(24):2540–9. [13] Paoletti E, Cannella G. Left ventricular hypertrophy in chronic kidney disease. G Ital Nefrol 2006;23(6):560–8. [14] Kaysen GA, Eiserich JP. The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol 2004;15(3):538–48. [15] Mann J, Hilger KF, Veelken R, Lenhardt C, Schiffrin EL, Chronic kidney disease and the cardiovascular system. Internist (Berl) 2008;49(4): 413–414, 416–418, 420–421. [16] London GM, Marchais SJ, Metivier F, Guerin AP. Cardiovascular risk in end-stage renal disease: vascular aspects. Nephrol Dial Transplant 2000;15(Suppl 5):97–104. 274 Please cite this article in press as: Soliman RA et al. Assessment of hypotension during dialysis as a manifestation of myocardial ischemia in Q1 patients with chronic renal failure, Egypt J Crit Care Med (2014), http://dx.doi.org/10.1016/j.ejccm.2014.05.001 268 269 270 271 272 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299 300 301 302 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 324 325 326 327 328 329 330