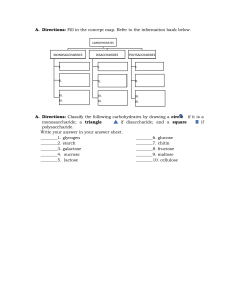
Lab Report Module 1 Conversion problems 1. 237 grams (g) is equal to 0.237 kilograms (kg). 2. 6.75 millimeters (mm) is equal to 0.00675 meters meters (m). 3. 0.34 liters (l) is equal to 340 milliliters (ml). 4. 1,735 centimeters (cm) is equal to 17.35 meters (m). 5. 0.025 kilograms (kg) is equal to 25,000 milligrams (mg). 6. 3456 nanometers (nm) is equal to 0.003456 millimeters (mm). 7. 0.0075 milliliters (ml) is equal to 7,5000 microliters (μl). 8. Jorge is working in a lab. The lead scientist has asked him to make up a solution containing 350 milligrams of dextrose. However, the scale only weighs in grams. How many grams of dextrose should Jorge put in the solution? 0.35 grams 9. Susan has been told she needs to take 3 grams of a drug everyday. Her pills only come in 250 milligram sizes. How many pills should she take every day? 12 pills SOLVE THE PH PROBLEMS 1. The pH of rainwater is about 5.6. Is rainwater acidic, basic, or neutral? acidic 2. The pH of a Coke is 2. What is the hydrogen ion concentration of the solution? 0.01 M 3. What is the hydroxide ion concentration of Coke (pH 2)? 1 x 10^-12 M 4. Solution A has a pH of 3, and solution B has a pH of 5. This means solution A is more acidic than solution B. This also means that solution A has more hydrogen ions than solution B. 5. Solution A has a pH of 12 and solution B has a pH of 7. This means solution A is more basic than solution B. This also means that solution A has fewer hydrogen ions than solution B. 6. You are creating a tank to grow tadpoles in for an experiment. You know that tadpoles prefer a relatively neutral pH. Over time the water is likely to become more acidic due to tadpole excretions and other biological processes. What might you add to the tank to maintain the pH near neutral? buffer solution Part 3 Interactive Lab Hypothesis: The addition of CO2 to ocean water using the straw will result in a decrease in the pH level. Experiment: 1. Fill a container with ocean water. 2. Place the pH meter into the water to get a baseline reading. 3. Blow bubbles into the ocean water using the straw for 5 minutes. 4. Remove the straw and place the pH meter back into the water to get a new reading. 5. Record the new pH reading. Results: The pH of the ocean water decreased from 8.1 to 7.6 after bubbling CO2 into it for 5 minutes. Effect of C02 on Ocean PH 3,2 Time (in mnutes) 2,7 0,8 0 1 2 3 4 5 6 7 8 9 10 Ph Level Conclusion: The hypothesis that the addition of CO2 to ocean water using the straw will result in a decrease in pH level is supported by the data. After bubbling CO2 into the ocean water for 5 minutes using the straw, the pH level decreased from 8.1 to 7.6. This supports the equation showing that CO2 reacts with water to form carbonic acid, which results in ocean acidification. Macromolecules Experiment Hypotheses: - Chicken will contain protein due to its muscle tissue. - Mashed potatoes will contain carbohydrates due to its starch content. - Sprite will contain carbohydrates (sugar) due to its sweet taste. Predictions: | Macromolecule | Chicken | Mashed Potatoes | Sprite | --- | --- | --- | --- | | Protein | Positive | Negative | Negative | | Carbohydrates | Negative | Positive | Positive | | Lipids | Negative | Negative | Negative | | Nucleic Acids | Negative | Negative | Negative | Results: | Macromolecule | Chicken | Mashed Potatoes | Sprite | | --- | --- | --- | --- | | Protein | Positive | Negative | Negative | | Carbohydrates | Negative | Positive | Positive | | Lipids | Negative | Negative | Negative | | Nucleic Acids | Negative | Negative | Negative | The chicken tested positive for protein, the mashed potatoes tested positive for carbohydrates, and the Sprite tested positive for carbohydrates. Our hypotheses regarding the presence of protein in chicken and carbohydrates in mashed potatoes and Sprite were correct. Our hypotheses regarding the absence of lipids and nucleic acids in all three unknowns were also correct. The absence of lipids and nucleic acids were not surprising as they are not typically found in large amounts in these types of foods. However, the lack of carbohydrates in the chicken was unexpected. This may have been due to the cooking process or the cut of chicken used in the experiment. It is also possible that there was a mistake in the testing process. Further experimentation would be needed to confirm these hypotheses.