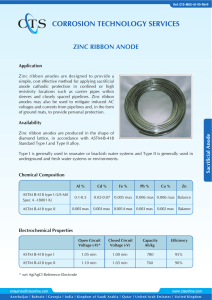
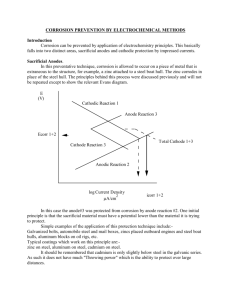
Chem 30 Electrochem Research Project Notes Muhammad Suleman Topic: Sacrificial Anodes Notes: (Be sure to record your sources with your notes, put your notes in your own words to avoid unintentional plagiarism, and update your notes regularly.) Explanations The sacrificial anode is a particular kind of cathodic protection system. The metal alloy used to create the anode has a higher "active" voltage (greater negative electrochemical potential) than the metal used to create the cathode, which is used to safeguard the structure. The sacrificial anode material corrodes in preference to the structure due to the difference in potential between the two metals. This successfully halts the oxidation reactions on the shielded structure's metal. The surface that will be shielded from corrosion is the parent surface. The more reactive the metal, the easier it will oxidize and act as a cathode, shielding the metallic complex. The anode is referred to as a sacrificial anode since it sacrifices itself for the other compound as it corrodes first. Sacrificial anodes operate on a similar theory to electrolysis, whereby, when an anode and a metallic strip are submerged in an electrolytic solution, anode electrons dissolve, deposit, and transform the metallic strip into a cathode. For the sacrificial anode approach to operate, there must be two additional requirements for the anode and cathode. For the electrons to travel from the anode to the material it is protecting, there must be a return current path (usually by physical contact) and an electrolyte (water, humidity) to transport the electrons. Magnesium, aluminum, and zinc are the three metals most frequently used in sacrificial anodes. Of the three, magnesium has the most negative electron potential. The use of sacrificial anodes prevents corrosion in refineries, above-ground tanks, subterranean tanks, pipelines, water heaters, ship hulls, and distribution systems. Cathodic protection systems for sacrificing anodes are necessary for routine inspections and the replacement of any anodes that have been consumed. Applications Wind Turbines Offshore wind turbines are vulnerable to rapid flow rates and changing seabeds because they are typically located in shallow water. Cathodic protection designs that are frequently utilized might not be sufficient in some cases. Sacrificial anodes or impressed current cathodic protection can be used to offer reliable cathodic protection for the outside surfaces of the base's submerged zone. Sacrificial anodes can be created in any shape or material in order to satisfy the needs of the majority of applications. The pictures below depict the most common anode models used to prevent corrosion on the external submerged portions of wind turbine foundations. Ships One such instance is metal in seawater, where the iron metal comes into touch with the electrolytes. In a typical situation, the iron metal would rust after reacting with the electrolytes, becoming weaker in structure, and dissolving. Zinc, a common choice for ship anodes, would be added to stop the iron metal from "corroding". In this instance, the zinc sacrificial anode is electrically connected to the ship's hull. Seawater functions as an electrolyte, transferring electrons from the anode to the steel plate by oxidizing it and creating a protective layer. Zinc's standard reduction potential is around -0.76 volts, as seen in the table of Standard Reduction Potentials. Iron has a typical reduction potential of approximately -0.44 volts. Due to their different reduction potentials, zinc and iron would oxidize at a significantly higher rate. In fact, zinc would entirely oxidize before iron would start to react, protecting the ship's core structure. Pipelines The main purpose of sacrificial anodes for buried pipelines is to provide short-term protection while the pipelines are being installed. Before a permanent ICCP system is turned on, magnesium sacrificial anodes are frequently used as temporary protection for underground pipelines during the construction and installation phase. Submerged pipelines can be shielded using magnesium sacrificial anodes with high driving potential. They are most frequently used when the soil resistivity and current demand are both low. Due to magnesium anodes' greater propensity to self-corrode than other anode materials, the service life is constrained. Sacrificial anodes are frequently used in existing pipelines with pipe sockets that do not have metal contact because of intermediate rubber gaskets. In these conditions, one anode is present in each pipe section. Impact Environmental Sacrificial anodes are frequently employed to prevent corrosion in submerged metallic structures. Even though structures are shielded from corrosion due to the sacrificial anode metal being applied, it is susceptible to disintegrating over time and being released into the ocean. After being released, the metal particles may settle in the local waters and affect the ecosystem. The overall process results in the dissolution of the zinc anodes, which contaminates the sediment and seawater. Throughout a 12-month saltwater experiment, the quantities of heavy metals (Cr, Cu, Pb, and Zn) in water and sediment samples were monitored to determine any potential environmental effects of continuous zinc dissolution. Sediment samples underwent a progressive extraction process in order to separate the zinc mobile fractions from the remaining ones. Zinc concentrations in water and surface sediments dramatically increased under the working conditions of sacrificial anodes benign applied. This showcased how zinc concentrations in water became another source of pollution. Economic According to a 2013 estimate, the global cost of corrosion is estimated to be US$2.5 trillion, or 3.4% of global GDP. By using modern corrosion control technology, such as sacrificial anodes, it is anticipated that between 15% and 35% of the cost of corrosion, or between US$375 and $875 billion annually on a global basis, might be saved. Environmental expenditures and costs related to personal safety are frequently left out of this calculation. Numerous sectors have understood how costly it can be to not manage corrosion and how poor corrosion control may result in significant profit loss over the course of an asset's lifetime. Question: What is The Significance of Sacrificial Anodes? Answer: The sacrifice anode is a very significant invention due to its efficiency in performing redox processes to shield other metals from corrosion. When a sacrificial anode has completely corroded, simply replace it with a new one to continue its functionality. Even though sacrificial anode corrosion does contribute to some marine water pollution, the money saved and greater pollution avoided by shielding the primary structural metal of various items far outweigh its drawbacks. References Anish. (2021, September 23). Understanding sacrificial anodes on ship. Marine Insight. https://www.marineinsight.com/tech/understanding-sacrificial-anodes-on-ships/ Cathodic protection: What is Cathodic Protection? CTL Corrosion Technologies. (2022, June 8). Retrieved June 20, 2022, from https://www.corrosiontechnologies.ca/what-is-cathodic-protection/ Economic impact. impact.nace.org. (n.d.). Retrieved June 20, 2022, from http://impact.nace.org/economic-impact.aspx Jamborowicz, M. (n.d.). The Boater's Guide to Corrosion. The Side-Power blog. Retrieved June 20, 2022, from https://blog.side-power.com/en/the-boaters-guide-to-corrosion Libretexts. (2020, August 15). Sacrificial anode. Chemistry LibreTexts. Retrieved June 20, 2022, from https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_( Analytical_Chemistry)/Electrochemistry/Exemplars/Corrosion/Sacrificial_Anode Rousseau, C., Baraud, F., Leleyter, L., & Gil, O. (2009, January 30). Cathodic protection by zinc sacrificial anodes: Impact on marine sediment metallic contamination. Journal of Hazardous Materials. Retrieved June 20, 2022, from https://www.sciencedirect.com/science/article/abs/pii/S0304389409001198 Sacrificial anodes for pipeline. Cathwell. (2019, December 6). Retrieved June 20, 2022, from https://cathwell.com/industries/pipelines/iccp/ Sacrificial anodes for wind turbine foundations. Cathwell. (2019, December 6). Retrieved June 20, 2022, from https://cathwell.com/industries/wind-turbines/cathodic-protection/ Schematic showing cathodic protection methods using sacrificial anode ... (n.d.). Retrieved June 20, 2022, from https://www.researchgate.net/figure/Schematic-showing-cathodicprotection-methods-using-sacrificial-anode-and-impressed_fig1_272661241 Wen, C.-C., Tsai, H.-J., Hsu, S.-Y., & Yeh, H.-C. (2020, July 30). Environmental impact assessment of sacrificial anode method in Taiwan strait. Journal of Environmental Protection. https://www.scirp.org/journal/paperinformation.aspx?paperid=102149#:~:text=The%20s acrificial%20anode%20metal%20will,%5B3%5D%20%5B4%5D.