LECTURE #5
Chapter 4 –Water Quality
(Hwk#4- pp.112-113 2,4,6,10,14)
Chemistry Review
•Compound – A combination of Elements
•Molecule – Smallest part of a Chemical compound
•organic vs. inorganic- complex molecules of
carbon in combinations with other elements is
organic(i.e. organic pertains to living organisms and
an exception would be CO2)
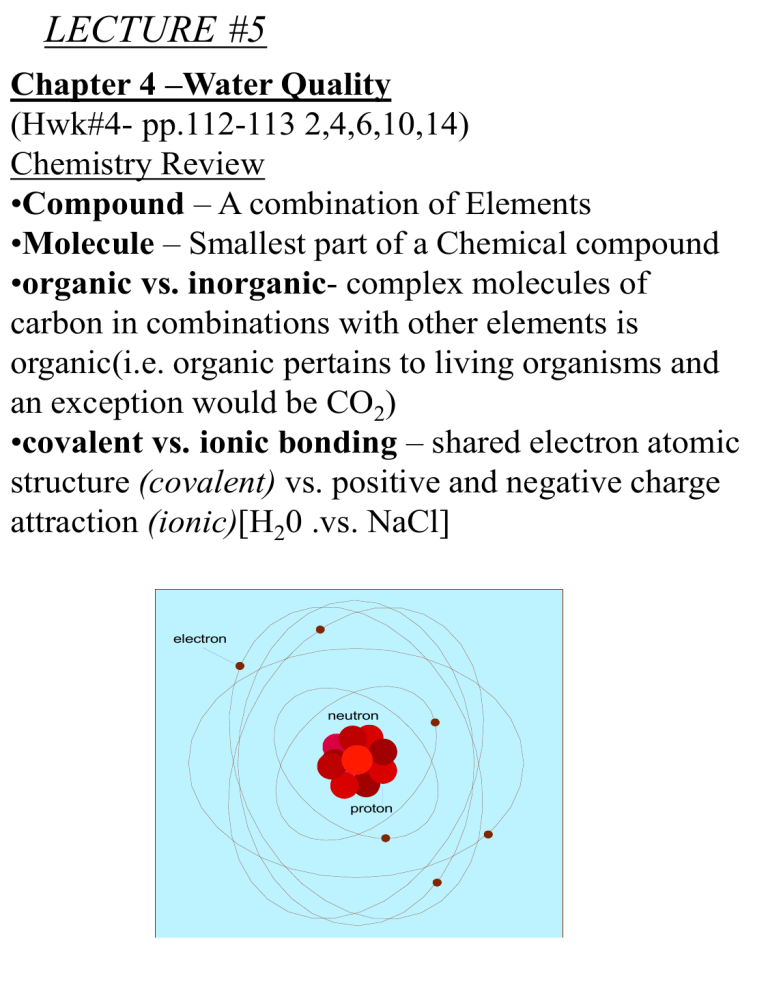
•covalent vs. ionic bonding – shared electron atomic
structure (covalent) vs. positive and negative charge
attraction (ionic)[H20 .vs. NaCl]
electron
neutron
proton
Chapter 4 –Water Quality
(Hwk#4- pp.121-122 2,4,6,10,14)
Chemistry Review
•Solutions – a uniform mixture of two substances
(solid, liquid, or gas)
•Solvent – the largest substance found in the solution
•Solutes – the smaller amount of the substances in a
solution
•Solubility Properties – Liquids (the warmer the
more soluble), Gas (the warmer the less soluble, the
lower the pressure, the lower the solubility)
[i.e. sugar in water, oxygen in water (table 4.3),
exception is alcohol in water]
Chemistry Review
•ionization– The dissociation or breaking apart of
molecules (to charged particles) when dissolved into
solution.[ NaCl Na Cl ]
•radicals – The dissociation of molecules into
groups of atoms. [ H 2O H OH ] OH is the
hydroxyl radical.
•Suspension vs. Colloids – Colloids are particles
less than coarse suspension but gt. than the true
solution [<0.1цm] and don’t settle in the solution.
[i.e. clay in water and fog in air are both colloids]
•Tyndall effect – the scattering of light in a solution
by colloids
Chemistry Review
•Flocculation– The clumping together of colloidal
particles by neutralizing the colloidal charges.
•dilute .vs. saturation – qualitative descriptions for
the concentration of solutes in a solution.
•Concentration Units – mg/L=1ppm,μg/L=1ppb,
1gpg=17.1 mg/L (for air - μg/m3) [1L of water
weighs 1kg therefore 1L is equivalent to 106mg],
i.e. 3.5%=35000mg/L, 1mg/L=8.34#/million gallons
Chemistry Review
•Acid – A substance that causes an increase in
hydrogen ions, H+
•Base- A substance that causes an increase in
hydroxyl radicals, OH•pH – a dimensionless scale that measures alkalinity
or acidic substance from 0 to 14.(the negative
logarithm of the hydrogen ion concentration)
[i.e. water numerical value of the hydrogen ion
concentration is ten to the minus seven. A ph of
5 is ten times more acidic than a ph of 6, because it is
logarithmic to the base 10-pp.90]
Chemistry Review
•Organic Substances– molecular structure {Carbon
plus some other elements}
•Organic compounds .vs. Organic chemicals
Hydrocarbons – contains only carbon and hydrogen
(i.e. Methane CH4[found in the decay process of
sewage and sludge], Benzene C6H6 [simplest ring
hydrocarbon]) pp.90-91
Organic chemicals (i.e. Alcohols) - Methanol
CH3OH,hydrogen is replaced by hydroxyl radicals.
i.e. solvents &fuel additives/Ethanol is a result of
the fermentation of sugar and is used for alcoholic
beverages.
H
H
c
H
H
H
H
c
H
OH
Chemistry Review
•Biodegradable – term for substances that can be
broken down by microbes into simpler molecules
•Carbohydrates –(contains C,O, and H) the basic
product of photosynthesis and are biodegadable
organic compounds.[i.e. glucose,sucrose (table
sugar),etc.]
•Starch and cellulose are more complex
carbohydrates
•fats are not very soluble in water
•Proteins – –(contains C,O, H,N and S) are formed
from amino acids are much more complex than
carbohydrates
Water Quality
•Turbidity– suspended particles which scatter light
and create a murky or cloudiness in the water
•Turbidity Unit (TU)–It is correlated to the
interference of light passage in 1 mg/L of suspended
silica. The nephelometers measure TUs. (TU<5 is
not noticeable)pp. 92
•Temperature – fish optimum temperatures ranges
between 15-32 degrees (i.e trout-15°, perch-24°,carp
–32°) note: increase in water temperature decrease
oxygen solubility.
•Color, taste, odor – important for drinkability.
i.e.hydrogen sulfide gas is produced from decaying
organic matter.
Threshold #- the amount of water required to
dilute the sample to remove the smell over the
original volume.
Chemical Parameters
•Dissolved Oxygen (DO)- is important for aquatic
life as well as land animals. ( 9ppm @20ºc in water)
•Biochemical Oxygen Demand (BOD)- The need
for oxygen when microbes consume the DO while
breaking down organic substances
Note: the higher the organic material the higher the
BOD.(expressed as mg/L of oxygen)
BODL-ultimate BOD or the maximum amount of
oxygen needed to biodegrade a given volume of
waste.(BOD>10mg/L has sewage pollution)
BODt BODL x(1 10 kt )
where k .15 / d @ 200 c, rate of BOD reaction
t time, d
BODt BOD at time t , mg / L
BODL BOD at the ultimate carbonaceous level , mg / L
( DO0 DO5 ) x300
V
where DO0 DO at time t 0
BOD5
DO5 DO at time t 5d
V sample volume, mL
Chemical Parameters
•Nitrification- when microorganism begin feeding
on noncarbonaceous ammonia after 8 to 10 days
•Chemical Oxygen Demand(COD)-it measures
all organics, including nonbiodegradable substances.
This test can not be correlated to the BOD test.
•Total Dissolved Solids(TDS)- The solids that
remain as a residue after evaporating the sample
from a dish
•Total Suspended Solids(TSS)- The solids that are
retained on the filter
TDS
([ wt of dish residue] wt of dish) x1000{in mg}
volume of sample filtered , mL
TSS
([ wt of filter solids] wt of filter ) x1000{in mg}
volume of sample filtered , mL
Chemical Parameters
•volatile solids- burns off at 550ºc
•Hardness-term to express the properties of highly
mineralized water (i.e. the concentration of Calcium
and Magnesium in water from soils and rocks)
all organics, including nonbiodegradable substances.
This test can not be correlated to the BOD test.
•Gas Chromotography/Mass Spectrometry-tests
for organics substances
•Atomic Absorption Spectrophotometers- detect
heavy metals
Name of
Substance
Iron, Manganese,
Copper, and Zinc
Flouride
Chlorine
Sulfates
Nitrogen
Phosphorus
Health Risk
none
good dental health
none - diluted
can form trihalomethanes
(carcinogen)
none
high levels of nitrates can
effect infants under 3 months
none
Acidity, Alkalinity,
and pH
none within normal
ph limits of 6-9.5
heavy metals
toxic -carcinogens leading to
death
radiation
toxic -due to unstable nuclei
emitting energy
Water Effects
bitter taste, turbid water, causes
staining
discolored teeth when consume
in excess
disinfectant and produce sanitary
waste.Can form Chloroform when
mixed with organics (precursors)
can create bad taste and laxative
effect. In sewage can form
Hydrogen Sulfide with a bad smell
promotes plants growth , such as
algae which degrades water quality
(eutrophication)
promotes plants growth , such as
algae which degrades water quality
(eutrophication)
elevated limits can cause bad
taste (acid are sour, bases are
bitter)
not always noticeable due to
a trace amount
not always noticeable due to
a trace amount
Name of
Micro-organism
Bacteria
Effects
definition
Through binary fission
they form colonies
-excessive growth reduces
sewage treatment efficiency
-autotrophic bacteria can feed
on simple inorganic compounds
-heterotrophic bacteria feed on
complex organic substances
-They are autotrophic
and can convert
inorganic matter into
organic using the sun
and chlorophyll
-They are called Phytoplankton
and produce 90% of oxygen
-They are a problem for public
water because of the taste and
odor problems.
-It is expensive to filter it out of the
water
aerobic and
anaerobic bacteria
They reproduce faster
(Can be filtered)
in warmer temperatures
algae
(can be filtered)
Protozoa
(can be filtered)
Viruses
(can not be filtered)
-The simplest of the
animal species (single
celled)
small pathogens which
take over a host cell
metabolic process for
their own use
-They are called zooplankton
-They can cause dysentery and
gastrointestinal disease
the cause of chicken pox,
rabies, yellow fever, polio,
colds, et.c
inactivation is done through
disinfection
Chemical Parameters
•coliforms- bacteria that is always present in the
human intestinal tract (E.coli)
[no coliform –no sewage – no pathogens]
Two methods for tesing:
Membrane Filter Method
Mulitple –Tube Fermentation Method





