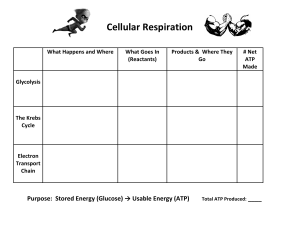
Chapter 19 Glycolysis based on Biochemistry, 4/e by Reginald Garrett and Charles Grisham All rights reserved. Requests for permission to make copies of any part of the work should be mailed to: Permissions Department, Harcourt Brace & Company, 6277 Sea Harbor Drive, Orlando, Florida 32887-6777 Outline 19.1 Overview of Glycolysis 19.2 Coupled Reactions in Glycolysis 19.3 First Phase of Glycolysis 19.4 Second Phase of Glycolysis 19.5 Metabolic Fates of NADH and Pyruvate 19.6 Anaerobic Pathways for Pyruvate 19.7 Energetic Elegance of Glycolysis 19.8 Other Substrates in Glycolysis GLYCOLYSIS Stepwise degradation of glucose Occurs in the cytosol Basically anaerobic process Principal steps occur with no requirement for oxygen First two billion years of biological evolution on earth Overview of Glycolysis (Embden-Meyerhof (Warburg) Pathway) Essentially all cells carry out glycolysis Ten reactions - same in all cells - but rates differ Two phases: First phase converts glucose to two G-3-P Second phase produces two pyruvates Products are pyruvate, ATP and NADH Three possible fates for pyruvate COUPLED REACTIONS (in anaerobic route of glycolysis) Phosphorylation To synthesize ATP using the metabolic free energy contained in the glucose molecule would be to convert glucose into one (or more) of the high-energy phosphates that have standard-state free energies of hydrolysis more negative than that of ATP. synthesized easily from glucose Phosphoenolpyruvate 1,3-bisphosphoglycerate acetyl phosphate. The first reaction phosphorylation of glucose Hexokinase or glucokinase This is a priming reaction - ATP is consumed here in order to get more later ATP makes the phosphorylation of glucose spontaneous Hexokinase 1st step in glycolysis ΔG large, negative (-33kJ/mol) under cellular conditions Hexokinase (and glucokinase) act to phosphorylate glucose and keep it in the cell Km for glucose is 0.1 mM; efficient at cell concentration of 4 mM glucose So hexokinase is normally active! Hexokinase is regulated - allosterically inhibited by (product) glucose-6-P - but is not the most important site of regulation of glycolysis - Why? Glucokinase (in the liver) 1st step in glycolysis ΔG large, negative (-33kJ/mol) under cellular conditions Glucokinase (Kmglucose = 10 mM) only turns on when the cell is rich in glucose Highly specific for glucose Hexokinase coverts to glucokinase at high glucose concentration. Inducible enzyme – the amount present is controlled by insulin Glucose-6-phosphate common to several metabolic pathways; hence, is a branch point in glucose metabolism Rx 2: Phosphoglucoisomerase Glucose-6-P to Fructose-6-P Why does this reaction occur?? next step (phosphorylation at C-1) would be tough for hemiacetal -OH, but easy for primary -OH isomerization activates C-3 for cleavage in aldolase reaction Ene-diol intermediate in this reaction Rx 3: Phosphofructokinase (PFK) PFK is the committed step in glycolysis! The second priming reaction of glycolysis Committed step and large, neg ΔG (-18.8 kJ/mol in erythrocytes) means PFK is highly regulated Most important site of regulation Rx 3: Phosphofructokinase (PFK) PFK increases activity when energy status is low PFK decreases activity when energy status is high Phosphofructokinase with ADP shown in white and fructose-6-P in red. ATP inhibits, AMP reverses inhibition Citrate is also an allosteric inhibitor Fructose-2,6-bisphosphate is allosteric activator FIGURE 19.8 ● At high [ATP], phosphofructokinase (PFK) behaves cooperatively, and the plot of enzyme activity versus [fructose-6-phosphate] is sigmoid. High [ATP] thus inhibits PFK, decreasing the enzyme’s affinity for fructose- 6phosphate. FIGURE 19.9 ● Fructose-2,6bisphosphate activates phosphofructokinase, increasing the affinity of the enzyme for fructose-6-phosphate and restoring the hyperbolic dependence of enzyme activity on substrate. FIGURE 19.10 ● Fructose-2,6bisphosphate decreases the inhibition of phosphofructokinase due to ATP. Rx 4: Aldolase C6 cleaves to 2 C3s (DHAP, Gly-3-P) Animal aldolases are Class I aldolases Class I aldolases form covalent Schiff base intermediate between substrate and active site lysine Rx 5: Triose Phosphate Isomerase DHAP converted to Gly-3-P An ene-diol mechanism Active site Glu acts as general base Triose phosphate isomerase is a near-perfect enzyme Catalytic perfection – turnover number near the diffusion limit Glycolysis - Second Phase Metabolic energy produces 4 ATP Net ATP yield for glycolysis is two ATP Second phase involves two very high energy phosphate intermediates 1,3 - BPG Phosphoenolpyruvate RxGly-3P 6: Gly-3-Dehydrogenase is oxidized to 1,3-BPG Energy yield from converting an aldehyde to a carboxylic acid is used to make 1,3-BPG and NADH Mechanism involves covalent catalysis and a nicotinamide coenzyme Formation of a covalent intermediate in the glyceraldehyde-3phosphate dehydrogenase reaction. Nucleophilic attack by a cysteine OSH group forms a covalent acylcysteine intermediate. Following hydride transfer to NAD, nucleophilic attack by phosphate yields the product, 1,3-bisphosphoglycerate. Rx 7: Phosphoglycerate Kinase ATP synthesis from a high-energy phosphate This is referred to as "substrate-level phosphorylation" 2,3-BPG (for hemoglobin) is made by circumventing the PGK reaction FIGURE 19.21 ● Formation and decomposition of 2,3-bisphosphoglycerate. Rx 8: Phosphoglycerate Mutase Phosphoryl group from C-3 to C-2 Rationale for this enzyme - repositions the phosphate to make PEP Note the phospho-histidine intermediates! Zelda Rose showed that a bit of 2,3-BPG is required to phosphorylate His Rx 9: Enolase 2-P-Gly to PEP Overall ∆G is 1.8 kJ/mol How can such a reaction create a PEP? "Energy content" of 2-PG and PEP are similar Enolase just rearranges to a form from which more energy can be released in hydrolysis Rx 10: Pyruvate Kinase PEP to Pyruvate makes ATP These two ATP (from one glucose) can be viewed as the "payoff" of glycolysis Large, negative ΔG - regulation! Allosterically activated by AMP, F-1,6-bisP Allosterically inhibited by ATP and acetyl-CoA Understand the keto-enol equilibrium of Pyruvate FIGURE 19.28 ● The conversion of phosphoenolpyruvate (PEP) to pyruvate may be viewed as involving two steps: phosphoryl transfer followed by an enol-keto tautomerization. The tautomerization is spontaneous (ΔG° = 35– 40 kJ/mol) and accounts for much of the free energy change for PEP hydrolysis. The Fate of NADH and Pyruvate Aerobic or anaerobic?? Pyruvate has two possible fates: aerobic: citric acid cycle anaerobic: LDH makes lactate The Fate of NADH and Pyruvate Aerobic or anaerobic?? NADH is energy - two possible fates: If O2 is available, NADH is re-oxidized in the electron transport pathway, making ATP in oxidative phosphorylation In anaerobic conditions, NADH is re-oxidized by lactate dehydrogenase (LDH), providing additional NAD+ for more glycolysis Energetics of Glycolysis The elegant evidence of regulation! Standard state ∆G values are scattered: + and • ∆G in cells is revealing: Most values near zero 3 of 10 Rxns have large, negative ∆ G negative ∆G rxns are sites of regulation! Large Other Substrates for Glycolysis Fructose, mannose and galactose Fructose and mannose are routed into glycolysis by fairly conventional means. Galactose is more interesting - the Leloir pathway "converts" galactose to glucose sort of.... Compartmentalization of glycolysis, the citric acid cycle, and oxidative phosphorylation.