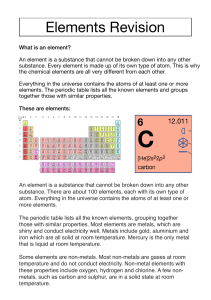
. Materials and their structure Describe the three states of matter as solid, liquid and gas in terms of the arrangement, separation and motion of particles. The three states of matter—solid, liquid, and gas—can be described in terms of the arrangement, separation, and motion of particles, providing insights into their distinct properties and behaviors: o Solid: Arrangement of Particles: In a solid, particles (atoms, ions, or molecules) are tightly packed and arranged in a regular, ordered pattern. They are held together by strong intermolecular forces or chemical bonds, which maintain a fixed, definite shape. Separation of Particles: The separation between particles in a solid is minimal. Particles are held in fixed positions and vibrate in place about their equilibrium positions. Motion of Particles: The motion of particles in a solid is restricted to small vibrations around their fixed positions. These vibrations increase with temperature but are not sufficient to allow the particles to move past one another easily. o Liquid: Arrangement of Particles: In a liquid, particles are close together, but they are not held in a rigid, ordered structure like solids. Instead, they have more freedom to move around one another. Separation of Particles: Particles in a liquid are relatively close together, but they have more separation compared to solids. They are able to flow and move past one another. Motion of Particles: Particles in a liquid have more kinetic energy than those in a solid. They are in constant motion, moving and sliding past each other. This motion allows liquids to take the shape of their container and flow. o Gas: Arrangement of Particles: In a gas, particles are widely separated and have no fixed order or structure. They are distributed randomly and fill the entire volume of their container. Separation of Particles: Particles in a gas have a significant separation between them, leading to a low density. They are free to move independently and are not constrained by forces to maintain any specific arrangement. Motion of Particles: Gas particles possess a high amount of kinetic energy. They move rapidly in all directions, colliding with each other and the walls of their container. This continuous, random motion allows gases to expand to fill their containers completely. These descriptions highlight the fundamental differences in the arrangement, separation, and motion of particles in solids, liquids, and gases. Solids have particles in a fixed arrangement with minimal separation and limited vibrational motion. Liquids have particles that are close together, allowing them to flow and move past each other. Gases have widely separated particles that move freely and independently, filling the entire volume of their container. These differences in particle behavior are responsible for the unique macroscopic properties of each state of matter. Describe a vacuum as a space devoid of matter. o o o o o o o o A vacuum, in the context of physics and science, is defined as a space devoid of matter, which means it contains little to no particles, atoms, molecules, or any other form of substance. In essence, it is a region of space where the pressure and density of matter are extremely low, approaching zero. This absence of matter results in several distinctive characteristics and effects: Lack of Air: A common example of a vacuum is the absence of air. In everyday terms, when we refer to a vacuum cleaner, we are essentially describing a device that creates a region of low pressure to suck in air and particles from its surroundings. Absence of Gases: In a vacuum, the concentration of gases, such as oxygen and nitrogen, is negligible. This makes it impossible for humans and most living organisms to survive without specialized equipment. No Sound Transmission: Sound requires a medium, typically air or another substance, to propagate. In a vacuum, where there is no matter to transmit vibrations, sound cannot travel. This is why space is often depicted as silent in science fiction movies. Lack of Heat Transfer: Heat transfer relies on the movement of particles (atoms or molecules) to transfer thermal energy. In a vacuum, where these particles are absent, heat transfer through conduction and convection is extremely limited. However, radiation (the transfer of heat via electromagnetic waves) can still occur in a vacuum. No Resistance to Motion: In a vacuum, there is no matter to provide resistance to the motion of objects. This means that, in the absence of gravitational forces or other external factors, objects can move freely and indefinitely at a constant velocity. Vacuum in Space: Outer space is often considered a near-perfect vacuum because it contains very few particles per unit volume. While it's not a complete vacuum, the low density of matter in space means that astronauts require specialized suits and equipment to survive and function. Vacuum Experiments: Vacuums are essential in scientific research and experiments. Researchers use vacuum chambers to create controlled environments with low pressure and density to study various phenomena, including material properties, chemical reactions, and the behavior of matter under extreme conditions. In summary, a vacuum is a region of space devoid of matter, characterized by a lack of gases, absence of sound transmission, limited heat transfer, no resistance to motion, and its importance in scientific investigations and space exploration. Understanding vacuums is crucial in many areas of science and technology. Understand that all matter is made of atoms, with each different type of atom being a different element. Understanding that all matter is made of atoms, with each different type of atom representing a different element, is a fundamental concept in chemistry and the physical sciences. This concept is often referred to as the "atomic theory" and was developed over centuries through the work of various scientists. Here are the key points to grasp: o Matter is Composed of Atoms: Atoms are the basic building blocks of matter. Everything around us, whether it's a solid, liquid, gas, or even a living organism, is made up of atoms. Atoms are incredibly small, and they cannot be subdivided further by chemical means. o Elements and Atoms: Elements are substances composed of only one type of atom. For example, oxygen is an element, and it consists of oxygen atoms (O). Each element is unique, and it is defined by the number of protons in the nucleus of its atoms. The periodic table of elements lists all known elements. o Different Types of Atoms: There are more than 100 different elements known to science, and each element is characterized by the type of atom it contains. These elements range from hydrogen (the lightest element) to uranium (a heavy element). Each element has its own unique properties. o Chemical Reactions and Compounds: Chemical reactions involve the rearrangement of atoms. When atoms of different elements combine in specific ratios, they form compounds. Compounds are substances composed of two or more different types of atoms bonded together. Water (H2O), for example, is a compound made of hydrogen and oxygen atoms. o Subatomic Particles: Atoms themselves are composed of subatomic particles, mainly protons, neutrons, and electrons. Protons and neutrons are located in the nucleus at the center of the atom, while electrons orbit the nucleus in energy levels or electron shells. o Atomic Number: The number of protons in the nucleus of an atom is called its atomic number. It is a unique identifier for each element. For example, all carbon atoms have six protons in their nucleus, so the atomic number of carbon is 6. o Isotopes: Atoms of the same element can have different numbers of neutrons, leading to variations known as isotopes. Isotopes of an element have the same number of protons but different atomic masses due to their differing numbers of neutrons. o Chemical Periodicity: Elements on the periodic table are arranged in a way that reflects their atomic structure and properties. The periodic table groups elements with similar properties together, making it a valuable tool for understanding and predicting chemical behavior. In summary, the concept that all matter is composed of atoms, with each element consisting of a unique type of atom, is a foundational principle in chemistry. It provides a framework for understanding the composition, behavior, and interactions of matter at the atomic and molecular levels, forming the basis for the study of chemistry and the physical sciences. Know that the Periodic Table presents the known elements in an order. The Periodic Table presents the known chemical elements in a systematic and organized order. This table is a fundamental tool in chemistry and provides valuable information about the properties and relationships of the elements. Here are some key points about the Periodic Table: o o o o o o o o o o Elemental Order: The elements in the Periodic Table are arranged in order of increasing atomic number. The atomic number of an element is the number of protons in the nucleus of its atoms and uniquely identifies each element. Rows and Columns: The Periodic Table is organized into rows and columns. Rows are called "periods," and there are seven of them. Columns are called "groups" or "families," and there are 18 numbered groups in total. Periods: Elements within the same period have the same number of electron shells or energy levels. As you move from left to right across a period, the atomic number increases, and the elements become progressively heavier and more complex in terms of their electron configuration. Groups: Elements within the same group share similar chemical properties. They have the same number of valence electrons, which are the electrons in the outermost energy level. The group number corresponds to the number of valence electrons. For example, elements in Group 1 (e.g., hydrogen, lithium, sodium) all have one valence electron. Block Classification: The Periodic Table is further divided into four blocks: sblock, p-block, d-block, and f-block, based on the types of orbitals being filled with electrons. The s- and p-block elements are the most commonly encountered in everyday chemistry. Color Coding: Elements in the Periodic Table are often color-coded to indicate their state at room temperature: solids, liquids, and gases. Additionally, some tables use colors to distinguish between different element categories, such as metals, nonmetals, and metalloids. Properties and Trends: The Periodic Table provides valuable information about the properties and trends of the elements. For example, elements in the same group tend to have similar chemical behaviors, and there are recurring trends in properties like atomic radius, electronegativity, and ionization energy as you move across periods and down groups. Transition Metals: The d-block elements (transition metals) are located in the center of the Periodic Table. They are known for their variable oxidation states and often form colorful compounds. Lanthanides and Actinides: The f-block elements, known as the lanthanides and actinides, are usually placed at the bottom of the Periodic Table to conserve space. These elements are often called the "rare earth elements" and include elements like uranium and thorium. The Periodic Table is a powerful tool that enables scientists and chemists to understand the relationships among the elements, predict their chemical behavior, and organize chemical knowledge. It has evolved over time as new elements have been discovered, and its structure continues to be a central reference point in the field of chemistry. Know metals and non-metals as the two main groupings of elements. Metals and non-metals are the two main groupings of elements in the Periodic Table, and they have distinct characteristics and properties that differentiate them from each other: Metals: o Conductivity: Metals are generally good conductors of electricity and heat. This is because they have a high density of free electrons that can move through the metal lattice and carry electrical current or thermal energy. o Malleability: Metals can be easily hammered or pressed into thin sheets without breaking. This property is known as malleability and is due to the ability of metal atoms to slide past each other in a regular pattern. o Ductility: Metals can be drawn into thin wires without breaking. This property is called ductility and is a result of the same ability of metal atoms to move and rearrange themselves without losing their metallic properties. o Luster: Most metals have a shiny, reflective surface when polished. This property is referred to as luster and is due to the ability of metal surfaces to reflect light. o Solid State at Room Temperature: The majority of metals are solid at room temperature, with the exception of mercury, which is a liquid at room temperature. o High Melting and Boiling Points: Metals generally have high melting and boiling points, meaning they remain in the solid state at high temperatures and require a significant amount of energy to melt or vaporize. o Tendency to Lose Electrons: Metals tend to lose electrons in chemical reactions, forming positively charged ions (cations). This is because they have few valence electrons and a strong desire to achieve a stable electron configuration by losing electrons. Non-Metals: o Poor Conductivity: Non-metals are typically poor conductors of electricity and heat. They have few or no free electrons available for conducting electricity, and their atomic structures do not facilitate the efficient transfer of thermal energy. o Brittleness: Non-metals are often brittle and break or shatter when subjected to stress. Unlike metals, their atomic structures do not allow for easy deformation. o Dull Appearance: Most non-metals have a dull or non-reflective appearance when in a solid state. o Various States at Room Temperature: Non-metals can exist in different states at room temperature. For example, some are solids (e.g., sulfur), some are gases (e.g., oxygen), and one (bromine) is a liquid. o Lower Melting and Boiling Points: Non-metals generally have lower melting and boiling points compared to metals, meaning they are often found in gaseous or liquid states at relatively low temperatures. o Tendency to Gain Electrons: Non-metals tend to gain electrons in chemical reactions, forming negatively charged ions (anions). They have a strong attraction for electrons to achieve a stable electron configuration. o Variety of Properties: Non-metals exhibit a wide range of properties, including being insulators, semiconductors, or highly reactive elements depending on their position in the Periodic Table. The classification of elements into metals and non-metals is a broad generalization that serves as a useful framework for understanding their basic properties. However, it's important to note that there are elements with intermediate properties known as metalloids (e.g., silicon, germanium) that exhibit some characteristics of both metals and non-metals. Use the particle model to represent elements, compounds and mixtures. The particle model is a useful conceptual framework for representing and understanding the structure of matter, including elements, compounds, and mixtures. It helps visualize how individual particles, such as atoms, ions, and molecules, come together to form different types of substances. Here's how the particle model can be used to represent these three categories: o o o Elements: In the particle model, an element is represented by a collection of identical atoms. Each atom is depicted as a single particle. For example, if we consider the element oxygen (O), the particle model would represent it as a group of oxygen atoms (O). The particles are typically shown as spheres or circles to emphasize their discrete nature. Elements are the simplest substances and consist of only one type of atom. Compounds: Compounds are represented in the particle model as a combination of different types of atoms bonded together to form molecules. For example, consider water (H2O). In the particle model, water is represented as a molecule consisting of two hydrogen atoms (H) bonded to one oxygen atom (O). The particles in compounds are shown as bonded units, often with lines or dashes between them to indicate the chemical bonds. Compounds have a fixed chemical formula that specifies the types and ratios of atoms present. Mixtures: Mixtures consist of different substances physically combined without forming new chemical bonds. The particle model for mixtures reflects this lack of chemical bonding. In a mixture, you would represent each substance with its respective particles. For example, a mixture of salt (NaCl) and sand (SiO2) would show salt particles and sand particles existing together without any chemical bonding between them. Mixtures can be further classified into homogeneous mixtures (solutions) and heterogeneous mixtures. In a homogeneous mixture like a saltwater solution, the particles are uniformly distributed. In a heterogeneous mixture like a salad with various ingredients, the particles are not uniformly distributed and can be seen as distinct components. The particle model helps visualize the composition and arrangement of particles in elements, compounds, and mixtures, aiding in the understanding of their physical and chemical properties. It is a fundamental concept in chemistry that serves as the basis for understanding various chemical reactions and processes. Describe the differences between elements, compounds and mixtures, including alloys as an example of a mixture. Elements, compounds, and mixtures are distinct categories of substances in chemistry, each with its own characteristics and properties. Alloys, such as bronze and steel, are examples of mixtures that contain metals. Here are the key differences between these three categories, along with a specific example of an alloy: o o o o Elements: Definition: Elements are pure substances composed of only one type of atom. They are the simplest form of matter and cannot be broken down into simpler substances by chemical means. Composition: Elements consist of a single type of particle, which is typically an atom of that element. For example, oxygen (O) is an element composed of oxygen atoms (O). Chemical Properties: Elements have unique chemical properties based on the characteristics of their atoms. These properties are determined by the arrangement and behavior of electrons in the atoms. Examples: Some common elements include hydrogen (H), carbon (C), nitrogen (N), and gold (Au). Compounds: Definition: Compounds are substances formed when two or more different elements chemically combine in fixed ratios. They are composed of molecules that contain multiple types of atoms bonded together. Composition: Compounds have a fixed chemical formula that specifies the types and ratios of atoms present in the molecule. For example, water (H2O) is a compound consisting of two hydrogen atoms (H) bonded to one oxygen atom (O). Chemical Properties: Compounds have distinct chemical properties that differ from those of the individual elements from which they are composed. Chemical reactions can break or form chemical bonds within compounds. Examples: Some common compounds include water (H2O), carbon dioxide (CO2), and sodium chloride (NaCl). Mixtures: Definition: Mixtures are combinations of two or more substances that are physically mixed together but not chemically bonded. The substances in a mixture retain their individual properties and can be separated by physical means. Composition: Mixtures can vary in composition, and the substances in a mixture can be present in any proportion. They do not have a fixed chemical formula. Physical Properties: Mixtures exhibit a range of physical properties depending on the substances involved. The properties of the components do not change in a mixture. Examples: Common examples of mixtures include air (a mixture of gases), salad (a mixture of various ingredients), and seawater (a mixture of water and dissolved salts). Alloys as Mixtures: Alloys are mixtures that specifically involve metals. They are created by blending two or more metallic elements together to enhance or modify their properties. Unlike compounds, alloys do not involve chemical bonding between the metal atoms; instead, the atoms are interspersed throughout the mixture. o • Example - Bronze: Bronze is an alloy made by combining copper (Cu) and tin (Sn). It is known for its strength, durability, and resistance to corrosion. In the particle model, bronze consists of a mixture of copper atoms and tin atoms that are evenly distributed throughout the alloy. Example - Steel: Steel is another common alloy, consisting mainly of iron (Fe) with small amounts of carbon (C) and other elements. It is renowned for its strength and versatility in construction and manufacturing. In summary, elements are composed of a single type of atom, compounds are formed by the chemical combination of different elements, and mixtures are physical combinations of two or more substances that retain their individual properties. Alloys are a specific type of mixture that involves metals and can exhibit unique properties not found in the individual metal elements.