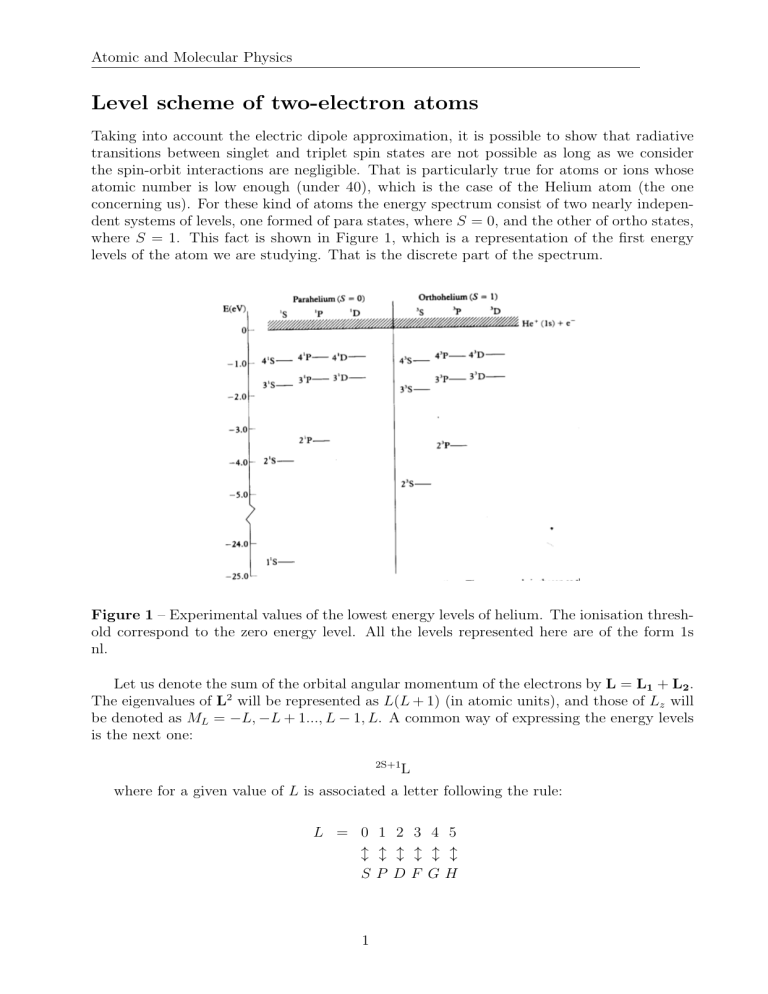
Atomic and Molecular Physics Level scheme of two-electron atoms Taking into account the electric dipole approximation, it is possible to show that radiative transitions between singlet and triplet spin states are not possible as long as we consider the spin-orbit interactions are negligible. That is particularly true for atoms or ions whose atomic number is low enough (under 40), which is the case of the Helium atom (the one concerning us). For these kind of atoms the energy spectrum consist of two nearly independent systems of levels, one formed of para states, where S = 0, and the other of ortho states, where S = 1. This fact is shown in Figure 1, which is a representation of the first energy levels of the atom we are studying. That is the discrete part of the spectrum. Figure 1 – Experimental values of the lowest energy levels of helium. The ionisation threshold correspond to the zero energy level. All the levels represented here are of the form 1s nl. Let us denote the sum of the orbital angular momentum of the electrons by L = L1 + L2 . The eigenvalues of L2 will be represented as L(L + 1) (in atomic units), and those of Lz will be denoted as ML = −L, −L + 1..., L − 1, L. A common way of expressing the energy levels is the next one: 2S+1 L where for a given value of L is associated a letter following the rule: L = 0 1 2 3 4 5 ↕ ↕ ↕ ↕ ↕ ↕ SP DF GH 1 Atomic and Molecular Physics It is easily deduced that the quantity 2S + 1, situated on the left, gives the multiplicity of the state, and takes the value 1 when S = 0 (singlet states), and 3 when S = 1 (triplet states). There are two interactions, which are the spin-orbit effect (concerning the interaction between the spin and the angular momentum) and the spin-spin effect (coming from the interaction of the spins of the electrons), whose direct consequence is the fine structure splitting of the levels shown before. Nevertheless, this fact cannot be appreciated in the previous figure. If we wanted to eliminate partially the degeneracy of the triplet states, we could define the total angular momentum as J = L + S (let us remind the eigenvalues of the J2 operator are J(J + 1) and those of Jz are Mz ), so that each energy level of the triplet states (unless the 3S state) splits into three different ones very close to each other, corresponding to the three different values of J = L − 1, L, L + 1. A full spectrum for the He++ nucleus and the two electrons is shown in the following figure. We choose the origin of the energy so that E=0 is the minimum energy necessary to ionize two electrons. Figure 2 – Complete energy level spectrum of helium. In contrast with the previous figure, the threshold for the ionisation of the two electrons is the new zero energy. In the diagram can be appreciated the discrete levels of helium are between −79.0eV (the energy of the ground state of He) and −54.4eV (the ground state of the He+ ). Consequently, we can establish that the ionisation potential for the helium ground state (the energy necessary for ionizing the atom) is, obviously, the difference of the energies of these two levels: Ip = E0 (He+ ) − E0 (He) = −54.4 + 79 = 24.6eV 2 (1) Atomic and Molecular Physics This spectrum is comparable for any other two-electron ions with an atomic number higher than two. Also we can consider the example of the hydrogen anion H − with only one bound state and a ionisation potential around 0.75eV , which tell us that this ion is not very stable and can easily dissociate into a neutral hydrogen atom and a free electron. Figure 3 – Ground state of H − . d⟨Nij ⟩ = αij dt ! X k̸=i ⟨Nik ⟩ + X ⟨Njk ⟩ k̸=j 3 − [(N − k − 1)αmin + kαmax ]⟨Nij ⟩ (2)




