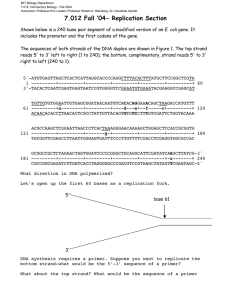
THE APPLICATIONS OF RECOMBINANT DNA Prepared by: Sir Cee Jae LESSON OBJECTIVES: give examples of products from recombinant DNA technology; illustrate the use of databases to search genes for desired traits; describe steps in PCR to amplify and detect a gene of interest; identify the parts of an expression vector; explain how genes may be cloned and expressed REVIEW ON CENTRAL DOGMA OF MOLECULAR BIOLOGY DNA (gene) => RNA (transcript) => Protein (trait) REVIEW ON CENTRAL DOGMA OF MOLECULAR BIOLOGY Different organisms have different traits based on their genes (DNA sequences). For example, frogs have antimicrobial peptides on their skin. Some jellyfish have proteins that allow them to glow in the dark. Mutations in hemoglobin genes lead to anemia. REVIEW ON CENTRAL DOGMA OF MOLECULAR BIOLOGY Based on the central dogma, if transcription and translation of genes lead to some traits, then the insertion of certain genes in a given organism may provide it with new traits. This is the basis for the development of genetically modified organisms (GMOs). THOUGHT EXPERIMENTATION DESIGNER GENE GROUP WORK CONSTRUCTING A GENETICALLY MODIFIED ORGANISM/TRAIT IN A FRUIT. I. Arrange the learners into groups of 4 or 5 II. Have them identify a special trait (e.g. large fruit size) III. Have them identify a source organism (e.g. jackfruit / langka) IV. Have them identify a target organism (e.g. aratilis) V. Have them identify the modified / added trait (e.g. langka-sized aratilis). VI. Have the learners present their work to the rest of the class, and let the class decide on the best proposal. DNA RECOMBINATION Modified Trait Insulin Production Gene Modification Insertion of Human Insulin Gene Recipient Organism Bacteria Application (Field) (Medicine) Production of Human Insulin in Bacteria DNA RECOMBINATION Modified Trait Pest Resistance Gene Modification Insertion of Bttoxin gene Recipient Organism Corn / Maize Application (Field) (Agriculture) Production of corn plants with increased resistance to corn boxer DNA RECOMBINATION Modified Trait Delayed Ripening Gene Modification Disruption of a gene for a ripening enzyme (e.g. polygalacturonase) Recipient Organism Tomato plant Application (Field) Agriculture) Production of plants with fruits that have delayed ripening fruits. These fruits will survive longer transport time, allowing their delivery to further locations (i.e. export deliveries) DNA RECOMBINATION Modified Trait Chymosin Production Gene Modification Insertion of a gene for chymosin Recipient Organism Bacteria Application (Field) (Industry) Enhance large scale production of chymosin. This enzyme serves as a substitute for rennet in the coagulation of milk. Rennet has to be harvested from calves. The large scale production of this enzyme in bacteria provides an abundant supply of this important component for the cheese production industry. DNA RECOMBINATION For example, one would want to find out if any work has been done on spider silks. The databases (e.g. Genbank:Nucleotide database) may be searched for entries that contain information on “Spiders, and Silk” (Result: 93615 entries). The results may be screened for more specific studies (e.g. Malaysia, Spiders, and Silk- Result two entries). PCR AMPLIFICATION Once a desired trait is chosen, information must be acquired for either its detection or expression in a given organism. DETECTION SOME RESEARCHERS MAY BE INTERESTED IN DETERMINING IF A GIVEN GENE/TRAIT IS AVAILABLE IN A PARTICULAR ORGANISM. IF NO PREVIOUS RESEARCH PROVIDES THIS INFORMATION, RESEARCHERS MAY TEST THE DNA OF DIFFERENT ORGANISMS FOR THE PRESENCE OF THESE SPECIFIC GENES. A TECHNIQUE THAT ALLOWS THE DETECTION OF SPECIFIC GENES IN TARGET ORGANISMS IS CALLED PCR. DETECTION PCR amplification is an in-vitro method that simulates DNA replication in vivo. It utilizes a thermostable (heat-resistant) DNA polymerase that builds single stranded DNA strands unto unwound DNA templates. PCR uses repeated cycles of incubation at different temperatures to promote the unwinding of the DNA template (~95°C); the annealing of a primer (a ~20bp oligonucleotide sequence (recall RNA primers in DNA replication) onto the ssDNA template strand (~54 - 60°C); and the extension of the generated ssDNA strand through the binding of complementary bases to the template strand (~72° C). DETECTION The thermostability of the polymerase allows it to survive the repeated cycles of denaturation, annealing and extension with little loss of enzyme function. Each cycle of PCR doubles the amount of the target sequence. A typical PCR experiment uses about 35 cycles of amplification. This increases the original amount of the target sequence by 235 (i.e. ~34 billion) times. DETECTION Gene detection by PCR involves the design of primers that would only bind to sequences that are specific to a target. For example, researchers would want to find out if gene X (e.g. the gene for insulin) is available in a target organism (e.g. a mouse, Mus musculus). Primers may be designed by looking at the available sequences for gene X in the databases (e.g. all the genes for insulin in different organisms; humans, pigs, cows, etc.). DETECTION The different gene X sequences must be aligned/ compared to match areas of sequence similarity (conserved sequences) and areas of sequence dissimilarity (non-conserved sequences). Primers designed to have the same sequence as the conserved areas will be specific for binding gene X sequences in all the target organisms. Primers designed to have the same sequence as the non-conserved areas will only be specific for the organisms which match its sequence. DETECTION Primers may be classified as forward or reverse primers. Forward primers are complementary and bind to the reverse complementary (non-coding) sequence of the gene. Reverse primers arecomplementary and bind to the coding sequence of the gene. STEPS IN PCR AMPLIFICATION Step 0: Undenatured Template ; Temp ~ 54 °"C; Template: double stranded (ds) DNA strand. Complementary sequences are held together by H-bonds 5’ A T GCGATGAGGATATGACCCGATAGATAGAGGTATCTAGAGAT 3’ (Coding strand) 3’ T A CGCTACTCCTATACTGGGCTATCTATCTCCATAGATCTCTA 5’ (Non-coding strand) Step 1: Template denaturation ; Temp ~ 95 °"C; Template: single stranded (ss) DNA strands; DNA strands are separated; H-bonds between complementary sequences are broken 5’ A T GCGATGAGGATATGACCCGATAGATAGAGGTATCTAGAGAT 3’ (Coding strand) 3’ T A CGCTACTCCTATACTGGGCTATCTATCTCCATAGATCTCTA 5’ (Non-coding strand) STEPS IN PCR AMPLIFICATION Step 2: Primer Annealing ; Temp ~ 54 °"C (dependent on primer melting temperature); Template: ssDNA strands. H-bonds are formed between complementary sequences on the primers and the target sequences. 5’ A T GCGATGAGGATATGACCCGATAGATAGAGGTATCTAGAGAT 3’ (Coding strand) Direction of elongation <<<<<<< CCATAGATC (Reverse Primer) 5’ GCGATGAGG 3’ >>>>>> Direction of elongation (Forward Primer) 3’ T A CGCTACTCCTATACTGGGCTATCTATCTCCATAGATCTCTA 5’ (Non-coding strand) STEPS IN PCR AMPLIFICATION Step 3: New DNA strand elongation ; Temp ~ 72 °"C; The two new dsDNA strands are formed by the elongation of the generated ssDNA and the H-bonds between the complementary sequences on these new strands and their templates. Each of the new dsDNA strands is made up of one old strand from the original template, and one new strand that was generated as a reverse complement of the template. This is called semiconservative replication of the sequence. New Strand 1: 5’ A T GCGATGAGGATATGACCCGATAGATAGAGGTATCTAGAGAT 3’ (Coding strand) (old) 3’ CGCTACTCCTATACTGGGCTATCTATCTCCATAGATC-5’ (Reverse Primer) (new) New Strand 2: 5’ GCGATGAGGATATGACCCGATAGATAGAGGTATCTAG-3’ (Forward Primer) (new) 3’ T A CGCTACTCCTATACTGGGCTATCTATCTCCATAGATCTCTA 5’ (Non-coding strand) (old) STEPS IN PCR AMPLIFICATION Step 4: Repeat step 1 to 3 for N number of cycles (N is usually 35) PCR Results The expected product of PCR amplification will depend on the sequences / position at which the primer sequences bind. If the forward primer starts binding at nucleotide 3 (coming from the 5’ end) of a 43bp long gene, and the reverse primer binds at a position complementary to nucleotide 39 of the coding strand, then a 37bp product is expected per cycle of PCR. STEPS IN PCR AMPLIFICATION Step 4: Repeat step 1 to 3 for N number of cycles (N is usually 35) STEPS IN PCR AMPLIFICATION Step 4: Repeat step 1 to 3 for N number of cycles (N is usually 35) PCR APPLICATION PCR may be used to detect the presence of a desired gene in an organism. Depending on the primer design, the expected product may represent only a specific region of the gene or the entire gene itself. The first case is useful for detection of the gene, or the detection of organisms with that specific gene within a sample. The second case is useful for the amplification of the entire gene for eventual expression in other organisms. The direct amplification/copying of a full gene is part of the process for “cloning” that gene. CLONING AND EXPRESSION SOME GENES PROVIDE ECONOMICALLY, AND INDUSTRIALLY IMPORTANT PRODUCTS (E.G. INSULIN-CODING GENES; GENES FOR COLLAGEN DEGRADATION). IN SOME CASES, SCIENTISTS WOULD WANT TO PUT THESE GENES INTO ORGANISMS FOR THE EXPRESSION OF THEIR PRODUCTS. ONE EXAMPLE WOULD BE THE INSERTION OF AN INSULINCODING GENE FROM THE HUMAN GENOME INTO BACTERIA. THIS ALLOWS THE “TRANSFORMED” BACTERIA TO NOW PRODUCE HUMAN INSULIN AS A PRODUCT. CLONING AND EXPRESSION Certain types of bacteria are capable of this process since they are able to take genes within their cell membranes for eventual expression. The genes are normally in the form of small, circular DNA structures called plasmids. The genes found in the inserted plasmid DNA sequence will be expressed as proteins that provide specific traits to the transformed bacteria. The basic components of an expression plasmid are listed in the following table. The purpose of each of these is also provided. CLONING AND EXPRESSION Component Promoter Purpose Allows the controlled expression of the desired gene in the presence of an inducing agent (e.g. beta- galactosidase; heat treatment (~65°"C) CLONING AND EXPRESSION Component Multiple Cloning Site Purpose DNA sequence or portion for the insertion of the desired gene. This section may contain sequences that will be cut by specific restriction endonucleases ( cuts within the molecule) If both the amplified gene and the plasmid are cut with the same restriction enzyme, then complementary sequences will be generated for each, allowing them to bind together or anneal. The desired gene is inserted into the multiple cloning site through this process. CLONING AND EXPRESSION Component Multiple Cloning Site Purpose CLONING AND EXPRESSION Component Purpose PCR Primers: 5’ GCGATGAGG 3’ (Forward Primer) 3’ CCATAGATC 5’ (Reverse Primer) Multiple Cloning Site Forward Primer + EcoRI target sequence: 5’ GAATTCGCGATGAGG 3’ Reverse Primer + EcoRI target sequence: 3’ CCATAGATCCTTAAG 5’ CLONING AND EXPRESSION Component Inserted Gene Sequence Purpose Successful insertion of a gene allows the expression of its protein product. This usually provides a specific trait to the “transformed” bacteria. For example, if the gene for Green Fluorescent Protein is placed within the expression plasmid, bacteria transformed with this plasmid will produce protein (GFP) that will allow the bacterial cells / colonies to glow green in the dark. CLONING AND EXPRESSION Component Antibiotic Resistance Gene Purpose Provides a way to screen a population of bacteria for those that took up the plasmid. For example, if an ampicillin resistance gene is encoded in the plasmid, then only bacteria which took up the plasmid will be able to grow on media with ampicillin. However, if the ampicillin resistance gene is cut and the gene is inserted here for cloning, then the cell will no longer be resistant to ampicillin. CLONING AND EXPRESSION Component Antibiotic Resistance Gene Purpose This is a way to select which among the colony of cells actually contain the inserted gene sequence. Bacterial cells whose ampicillin resistance gene have been cut will die in the presence (agar plate) of ampicillin. THINK FOR A MOMENT: GIVE OTHER HYPOTHETICALLY MODIFIED OR GENETICALLY ENGINEERED PLANTS AND ANIMALS WHICH CAN BE USED FOR HEALTH, INDUSTRY, AGRICULTURE AND FOR THE PROTECTION OF THE ENVIRONMENT. NOTE: CAUTION!!! Certain ethical principles should be followed and adhered to in the production of genetically modified organisms. Animal welfare should be taken cared of and human cloning must never be conducted. THINK FOR A MOMENT: Discuss how PCR may be used for the detection of disease causing pathogens in a population. Discuss how the cloning and expression of certain genes allows for massive production of the desired product.