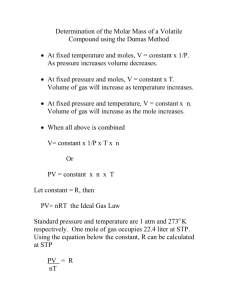
Simple Distillation: Recycle waste from biodiesel production • Boiling point composition of a mixture of cyclohexane and toluene Question: How far should you insert the thermometer into the distillation head? Answer: The thermometer should extend just below the side arm connecting the condenser to the distillation head. • You are measuring the temperature of the vapor that condenses in the condenser, you want the thermometer bulb located just below the side arm. Water • You always want the water to flow into the bottom of the condenser. It is because water will flow up, against gravity, and completely fill the condenser’s outer jacket. • If you were to add water at the top of the condenser, the water would fall to the bottom of the condenser and you would see an air pocket in the cooling jacket. To use a boiling point composition curve, you first need to determine the mole percent of one of your compounds in the two-component mixture. Set-Up Simple Distillation • You are heating the mixture so the temperature increases. The temperature will increase until it hits the liquid composition line. This is the temperature at which the mixture will begin to boil. Question: At what mole percent water should be in the first drop collected? Boiling Point Composition Curve • A boiling point composition curve of a twocomponent mixture has temperature on the y-axis and mole percent of one of the components on the x-axis. • The lowest curve on a boiling point composition diagram is called the liquid composition line. The highest curve is called the vapor composition line. This line tells you the mole percent of the compound when they are in the vapor phase at a given temperature. Answer: 100% mole percent water The vapor consists of 100% water because water’s boiling point (100 c) is so much lower than glycerol’s boiling point (290 c). Question: When you collect distillate in the collection flask, what happens to the original mole percent of water in the distillation flask? Answer: Mole percent of water decreases. The percentage of water, the lower boiling point compound, is higher in the vapor phase. Question: When you collected 50 ml of distillate in the collection flask, the mole percent of water in the distillation flask decreased to approximately 20 mole percent. With your distillation set-up, what percentage of water will be in the vapor phase at this position of the liquid? Answer: 98% mole percent water The mole percent water in the vapor is still very high because there is such a big difference between the boiling points of water and glycerol. • • When a compound boils, the temperature remains constant. Since water boils at 100 c, you can see water being distilled from the mixture as a flat line at that temperature. For a higher boiling point, the place where the solution is being removed is the highest temperature flat line on the temperature versus volume plot. Question: Where should you ideally change collection flasks during the distillation process to get one flask with pure water and one flask with pure glycerol? You want to change during the transition between the two boiling points. 3 mole methanol ≈ 2 moles water Question: How does it differ from the glycerol-water temperature vs volume plot that you observed in you first experiment? Answer: The boiling points of the two compounds are not as transparent and there is no defined transition between the two boiling points. Question: At what temperature do you expect the 60 mole percent methanol in water mixture to boil? Answer: 72% The liquid mixture in the flask should begin to boil at 72 c if you are performing the experiment at sea level. Remember, boiling point composition curves typically assume an atmospheric pressure of 760 mm Hg. Question: When you are performing this same distillation at a higher elevation for example, at 300 meters above sea level, how do you think the initial boiling point of the mixture in the distillation flask would change? Answer: The boiling point would decrease as compared to the boiling point at sea level. Question: If you collected the first drop from the distillation, what would be the mole percent methanol in that drop? Remember your, original distillation flask contains 60 mole percent methanol. Answer: 83 mole percent You are getting some separation of water and methanol, but the methanol is n0t 100% pure. Question: If the mole percent of methanol in the liquid phase in the distillation flask drops to 30%, what would be the mole percent of the methanol collected in the collection flask? Answer: 67 percent mole percent methanol As the mole percent of methanol decreases in the distillation flask, the mole percent of methanol also decreases in the collection flask.