Statistical Thermodynamics Homework - University of Minnesota
advertisement
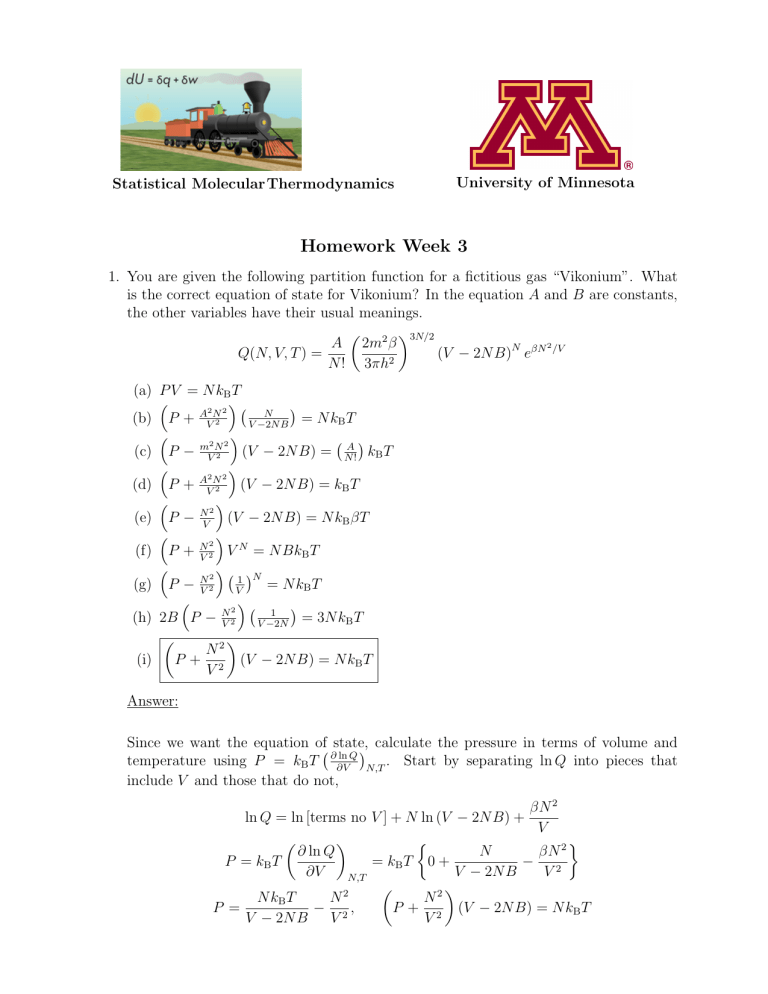
University of Minnesota Statistical Molecular Thermodynamics Homework Week 3 1. You are given the following partition function for a fictitious gas “Vikonium”. What is the correct equation of state for Vikonium? In the equation A and B are constants, the other variables have their usual meanings. 3N/2 A 2m2 β 2 (V − 2N B)N eβN /V Q(N, V, T ) = 2 N ! 3πh (a) P V = N kB T A2 N 2 N (b) P + V 2 = N kB T V −2N B 2 2 (c) P − mV N2 (V − 2N B) = NA! kB T 2 2 (d) P + AVN2 (V − 2N B) = kB T N2 (e) P − V (V − 2N B) = N kB βT 2 (f) P + N V N = N BkB T V2 2 1 N (g) P − N = N kB T 2 V V 2 1 (h) 2B P − N = 3N kB T 2 V V −2N N2 (i) P + 2 (V − 2N B) = N kB T V Answer: Since we want the equation of state, calculate the pressure in terms of volume and ∂ ln Q temperature using P = kB T ∂V N,T . Start by separating ln Q into pieces that include V and those that do not, ln Q = ln [terms no V ] + N ln (V − 2N B) + P = kB T ∂ ln Q ∂V P = N βN 2 = kB T 0 + − 2 V − 2N B V N2 P + 2 (V − 2N B) = N kB T V N,T 2 N kB T N − 2, V − 2N B V βN 2 V 2. In an Einstein crystal, one principal assumption is that each of the N atoms of the crystal vibrate independently about their lattice positions. The crystal is thus pictured in three dimensions as N independent three dimensional harmonic oscillators. Using the partition function for a harmonic oscillator, qho (T ) = ∞ X e −β(j+ 12 )hν −βhν/2 =e j=0 ∞ X e−βjhν , j=0 determine the partition function for an Einstein crystal. It will be helpful to make use of the geometric series, ∞ X xj = j=0 1 1−x to derive this result. −βhν/2 3 e (a) Q = 1−e −βhν (b) Q = (c) Q = e−βhν/2 1−e−βhν 3N e−βhν/2 1−e−βhν (d) Q = (e) Q = e−βhν/2 1+e−βhν (f) Q = eβhν/2 1+e−βhν 1 3N 1−e−βhν 3N 3 Answer: The partition function for a one-dimensional harmonic oscillator is given as qho (T ) = ∞ X e −β(j+ 21 )hν −βhν/2 =e j=0 ∞ X e−βjhν j=0 If we let x = e−βhν we can simplify the partition function to qho (T ) = e−βhν/2 ∞ X e−βjhν = e−βhν/2 j=0 j=0 Note that this summation is the geometric series, ∞ X j=0 xj = ∞ X 1 . 1−x xj We can then write the partition function as qho (T ) = e −βhν/2 ∞ X xj = e−βhν/2 j=0 qho (T ) = 1 1−x e−βhν/2 1 − e−βhν To find the partition function for a three-dimensional harmonic oscillator, think of the three-dimensional harmonic oscillator as three independent one-dimensional harmonic oscillators. The partition function for a three-dimensional harmonic oscillator can then be written as −βhν/2 3 e 3 q3D (T ) = [qho (T )] = 1 − e−βhν Each particle in the system is distinguishable. Therefore, the partition function for a system of N particles can be written as Q = [q3D (T )]N Substituting the partition function for the three-dimensional oscillator gives: Q= e−βhν/2 1 − e−βhν 3N N ) 3. One of the requirements for the statistical result that Q(N, V, T ) = q(V,T is that N! the system have many more available states than particles. Usually, the number of translational states alone are enough to satisfy this condition. Use the translational partition function to determine which of the following conditions leads to an increased number of accessible translational states: (a) small molar volume (b) low temperature (c) small mass (d) all of the above (e) none of the above Answer: In lecture video 3.6 it was discussed that the condition under which the number of available states exceeds the number of particles is N V h2 8mkB T 3/2 1 Large molar volumes, high temperatures, and large masses favor this condition, so the answer is none of the above. 4. The average energy for a monatomic van der Waals gas is 3 aNA2 hĒi = NA kB T − 2 V Use the definition of the constant volume molar heat capacity discussed in lecture video 3.4 to determine a formula for the constant volume molar heat capacity of a monatomic van der Waals gas. Here, NA is Avogadro’s number and R is the universal gas constant. (a) C̄V = 23 NA kB aN 2 (b) C̄V = 23 NA kB T − V A (c) C̄V = 23 NA kB − aNA2 aN 2 3 (d) C̄V = 2R − VA (e) C̄V = 23 R (f) C̄V = 43 RT 2 (g) C̄V = 34 NA kB T 2 − 2T aNA V (h) a) and e) (i) b) and g) (j) none of the above Answer: The constant-volume molar heat capacity is defined in lecture video 3.4 as ∂hĒi ∂ Ū = C̄V = ∂T N,V ∂T N,V Insert the average energy that is given 3 aNA2 hĒi = NA kB T − 2 V into the expression for the heat capacity: ∂hĒi ∂ 3 aNA2 C̄V = = NA kB T − ∂T N,V ∂T 2 V Differentiating with respect to temperature gives the heat capacity for a monatomic van der Waals gas as 3 C̄V = NA kB 2 Substituting R = NA kB , we can also see that 3 C̄V = R 2 This heat capacity is the same as that for a monatomic ideal gas. 5. In the lecture videos, a general formula for the average energy, ∂ ln Q 2 hEi = kB T , ∂T N,V was derived. Determine the average energy, hEi, for a monatomic ideal gas given the partition function for this gas, 1 Q(N, V, T ) = N! (a) hEi = (3/4)N kB T 2 + (b) 2πmkB T h2 3N/2 V N. N V hEi = (3/2)N kB T (c) hEi = (3/4)N kB T 2 (d) hEi = (3/2)N kB T + N V (e) hEi = (3/4)N kB T + 3N 2 (f) hEi = h2 2πmKB + N V 3N 2T Answer: First, rearrange this equation, 1 Q(N, V, T ) = T 3N/2 N! 2πmkB h2 3N/2 V N. Take the natural log of both sides of the partition function and separate the terms to obtain 3N 2πmkB 3N ln T + ln + N lnV ln Q(N, V, T ) = −ln N ! + 2 2 h2 Note that only the second term in the above equation depends on T . Therefore, differentiating with respect to T produces the following: ∂ ln Q ∂T = N,V 3N 2T Insert this result into the equation for hEi given above: ∂ ln Q 3N 2 2 hEi = kB T = kB T ∂T 2T N,V 3 hEi = N kB T 2 6. We found that under certain conditions, the partition function for a collection of particles, Q(N, V, T ), could be written in terms of the partition function for individual particles, q(V, T ), and the number of particles, N , Q(N, V, T ) = q(V, T )N . N! This equation is correct for a gas described by the van der Waals equation of state. (a) TRUE (b) FALSE Answer: We actually accept either answer as correct. The formula given is for a partition function involving particles that are indistinguishable, and all of which have identical and independent energy levels, because they do not interact with one another. In a van der Waals gas, the particles are indistinguishable, but the particles do interact with one another (as measured by the a and b parameters), so each particle’s energy depends upon its interactions with all other particles, making the given partition function equation inappropriate. However, note that the van der Waals gas partition function, Q, given in problem 1 of this homework can be rearranged so as to be written in the form Q(V, T ) = q(V, T )N . N! While not derived here, this comes from assuming a uniform distribution of particles which generates a ”mean-field” potential energy with which each particle interacts ”independently”, in which case use of the non-interacting ensemble partition function can be viewed as justified. Clearly, the assumptions are not really internally consistent, since you can’t both interact and not interact simultaneously! But, that is how the van der Waals equation of state is derived. 7. For a system that has equally spaced non-degenerate energy levels, at a fixed temperature what will happen to the value of the partition function, Q(N, V, T ), if the spacing between the energy levels INCREASES? (a) Q will increase (b) Q will decrease (c) Q will remain the same (d) either (a) or (b) depending on the temperature (e) none of the above Answer: See the plots in lecture video 3.2. If the temperature is fixed, and the spacing between the non-degenerate energy levels increases, then there are fewer states available to the system at that given temperature. Therefore, the partition function will decrease. 8. The following expresses the partition function of the system in terms of the partition functions of the individual particles (such as atoms or molecules), Q= qN . N! For this to be true, it must be the case that there are many more particles than available states. (a) TRUE (b) FALSE Answer: We had to assume that the number of available states was greater than the number of particles. Therefore the answer is false. 9. Derive that the fraction of harmonic oscillators in the ground vibrational state is given by f0 = 1 − e−hν/kB T . Then, given that ν̃ for N2 is 2330 cm−1 , calculate f0 for N2 (g) at 300 K and 1000 K. (a) 1.0000 and 0.7457 (b) 0.9000 and 1.0000 (c) 1.0000 and 0.9650 (d) 1.0000 and 0.0000 (e) 0.9578 and 0.8762 (f) 0.9200 and 0.4578 Answer: To find an expression for the probability that a harmonic oscillator will be found in the jth state, start with the general equation for the probability pj = e−βEj qvib where Ej = hν(j + 12 ) for a harmonic oscillator. Substituting into the equation for the probability 1 e−βEj e−βhν(j+ 2 ) e−βhνj e−βhν/2 pj = = = qvib qvib qvib We have shown that the partition function for the harmonic oscillator is qvib = e−βhν/2 1 − e−βhν Insert this into the equation for the probability pj = e−βhνj e−βhν/2 e−βhν/2 1−e−βhν = e−βhνj e−βhν/2 (1 − e−βhν ) = e−βhνj (1 − e−βhν ) e−βhν/2 From the preceding equation we can see that for the ground state (j=0), p0 = e−βhνj (1 − e−βhν ) = 1 − e−βhν Therefore, the fraction in the ground state is equal to this probability. f0 = p0 = 1 − e−βhν = 1 − e−hν/kB T To find the fraction of harmonic oscillators in the ground state at different temperatures, first convert the fundamental vibrational frequency for N2 to s−1 . For ν̃ = 2330 cm−1 . ν= c = cν̃ = (3 × 1010 cm · s−1 )(2330 cm−1 ) = 6.99 × 1013 s−1 λ We can then obtain a value for hν/kB : hν (6.626 × 10−34 J · s)(6.99 × 1013 s−1 ) = = 3352 K kB 1.38 × 10−23 J · K−1 This simplifies the equation for the fraction of harmonic oscillators to: f0 = 1 − e−hν/kB T = 1 − e(−3552 K)/T Insert the different temperatures for T to obtain the fraction in the ground state: Temperature (K) f0 300 1.000 1000 0.9650 10. Imagine you are given one mole of a homonuclear diatomic molecule, X2 , at a fixed volume and temperature. If you increase the mass of the molecules, for example you go from F2 to I2 , the partition function will, (a) increase (b) decrease (c) not change Answer: Inspection of the various component partition functions reveals that as the mass goes up, the translational, rotational, and vibrational levels all get closer together. At a fixed temperature this means that the partition function will increase.