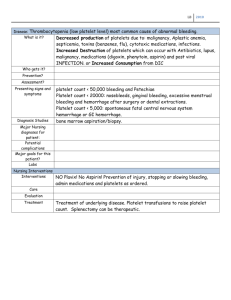
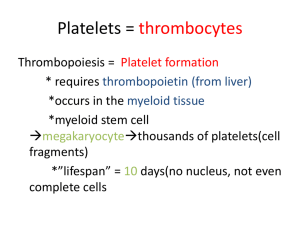
Lecture 1 Hemostasis HEMO= Blood, STASIS= Stop Complex physiologic process that keeps circulating blood in a fluid state Maintains balance between: Coagulation (clotting) & Hemorrhage (bleeding) When an injury occurs: Produces a clot to stop the bleeding Confines the clot to the site of injury Dissolves the clot as the wound heals Components of the system have: Procoagulant properties- initiate clotting if required Anticoagulant properties- prevent undesirable clotting Fibrinolytic/Fibrinolysis properties- clot breakdown The balance between bleeding & clotting is ensured by a complex cascade which can be divided in three interacting processes: 1. Primary Hemostasis: Formation of Platelet plug 2. Secondary Hemostasis: Formation of Fibrin clot 3. Fibrinolysis: Break down of the Fibrin clot Hemostasis involves the interaction of the following components to stop bleeding: • Blood vessels- contain Tissue Factor and undergo vasoconstriction • Platelets- platelet adhesion, aggregation and secretion • Plasma components- zymogens, cofactors, control proteins, and fibrinogen • Fibrinolytic proteins - fibrin clot breakdown • Inhibitors – inhibition of coagulation Blood Vessels Circulate blood throughout body Structured into three layers Inner layer- Tunica intima Middle layer- Tunica media (M) Outer layer- Tunica adventitia (A) Inner BV Layer- Vascular Endothelium Releases variety of proteins based on need: Anticoagulant – normally to prevent spontaneous clotting or when clot is formed Procoagulant – when there is injury Fibrinolytic – involved in clot dissociation Middle BV Layer Depending on BV type and size, contains muscle Smooth muscle cells contract = Vasoconstriction Outer BV Layer Sub-endothelium Support a surface protein called Tissue Factor Procoagulant- Important for the INITIATION of coagulation Red Blood cells • Add bulk & structural integrity to final fibrin clot White blood cells • Help stimulate wound healing • Monos & Lymphs have Tissue Factor on surface that can trigger coagulation Platelets • Procoagulant only- initiate and control hemostasis • PLTs do not prevent clotting or break down the clot, instead they adhere, aggregate, and secrete their granule contents to help form a clot PLTs are produced by megakaryocytes (MKs) through endomitosis • MK: largest cells in the BM (30-50μm), multilobulated nucleus, hypergranular cytoplasm, polyploid (multiple copies of chromosomes) • MK: derived from CFU-GEMM under the influence of IL-3, TPO and Meg-CSF Megakaryocytic Progenitors: • BFU-Meg clones hundreds of daughter cells • CFU-Meg clones a dozen daughter cells • LD-CFU-Meg undergoes the first stage of endomitosis ENDOMITOSIS DNA is duplicated without cell division MKs become ‘polyploid’ o Most have a ploidy of 16N (range 4N - 64N) MKs are the largest normally occurring cells in the marrow (30-100mm) o Only polyploid cells in body Megakaryocyte progenitors enter terminal differentiation stages as proliferation continues o More DNA= larger cell or more cytoplasm synthesized= more PLTs MK 8N or 16N can produce 2000-4000 platelet THROMBOPOIESIS Massive amounts of DNA produce equally massive amounts of cytoplasm & proteins The plasma membrane ‘invades’ the cytoplasm in a series of channels – this is the Demarcation System or DMS The DMS is biologically identical to MK plasma membrane and form basis for fragmentation into single PLTs The DMS dilates & tubules called ‘proplatelet processes’ develop These squeeze through or between endothelial cells and break off or shed into the central vein of BM After the cytoplasm is released as platelets the MK nucleus is reabsorbed by macrophage MARKERS FOR IDENTIFICATION OF MK AND PLT MPL: expressed in all maturation stages CD34: only on MK progenitors CD41: all positive except BFU-Meg, αIIb portion of αIIbβ3 (GPIIb/IIIa, CD41/CD61) CD42: MKI through PLT positive, GPIb portion of VWF receptor (GPIb/IX) PF4: MKI through PLT positive VWF: MKI through PLT positive, by immunostaining Fibrinogen: only MKIII and PLT positive, by immunostaining CONTROL OF THROMBOCYTOPOIESIS Thrombopoietin or TPO Hormone produced by kidney • Also produced by liver and smooth muscle cells Attaches to circulating platelets via membrane receptor PLTs = circulating TPO • Stimulates platelet production Thrombopoietin induces: • Stem cells to differentiate into MK progenitors • MK progenitors to differentiate into MK • Proliferation and maturation of MK • Platelet release from MK PLT STRUCTURE Membrane structure SCCS DTS PLT granules PLTs have plasma membrane receptors for: ADP Serotonin Collagen (GP VI, GPIa/IIa) vWF (GP Ib/IX/V) Fibrinogen (GP IIb/IIIa) Thrombin Epinephrine PLT GRANULES α granules, 50-80/plt (contain many proteins): o Fibrinogen o Factor V o vWF o β-thromboglobulin o HMWK o PAI-1 o Plasminogen Dense granules, 2-7/plt: o ADP, ATP, serotonin, Ca2+, Mg2+ PLT FUNCTION Complex, metabolically active cells that interact with their environment Initiate and control hemostasis At the time of an injury, platelets: o o o Adhere to site of injury/disruption (Adhesion) Aggregate to with other (Aggregation) Secrete their granule contents (Secretion) Platelet Function: At Sites of Injury Adhesion: • PLTs bind collagen directly through GPVI and GPIa/IIa or indirectly through vWF (which binds GPIb/IX/V on PLTs), ADP, TXA2, “inside-out”, “outside-in” signaling Aggregation (key step): • PLTs bind to each other through GPIIb/IIIa, shape change, membrane PL flip-flopping Secretion: • PLTs release granular contents (α and dense) • Mostly coag proteins • Launching platform for secondary hemostasis PRIMARY HEMOSTATIS DISORDERS Either PLT or Vascular abnormalities: QUALITATIVE or QUANTITATIVE Congenital or Acquired Manifest as mucocutaneous bleeding o Common Presentation: Bruising Petechiae Purpura Ecchymosis Epistaxis Gingival bleeding THROMBOCYTOSIS Reactive thrombocytosis – secondary to other conditions For example, post-surgery, post-splenectomy, IDA, inflammation or disease, strenuous exercise Not associated with thrombosis or hemorrhage Disappears when underlying condition under control Myeloproliferative disorders For example, Essential Thrombocythemia, Polycythemia Vera, CML, or Primary Myelofibrosis Can cause either thrombosis or bleeding Marked and persistent elevations in PLT count are seen THROMBOCYTOPENIA Characterized by Platelet count of < 150 x 10^9/L Most common cause of clinically significant bleeding: <100 x 10^9/L Pathophysiologic processes that result in Thrombocytopenia: 1. Impaired or Decreased platelet production: Lack of adequate bone marrow megakaryocytes Ineffective Thrombopoiesis 2. Increased/Accelerated platelet destruction Caused by immunologic responses Caused by mechanical damage, consumption, or sequestration 3. Abnormal platelet distribution or dilution Splenic sequestration due to splenomegaly- abnormal sequestration IMMUNE THROMBOCYTOPENIC PURPURA Destruction of platelets by an antibody-mediated mechanism (Ab against GPII/IIIa, GPIb/IX/V, GPIa/Iia) In children (2-5 ys old) following viral infections or vaccinations (MMR, DTP, polio) Key hematologic feature is thrombocytopenia (3-4% cases: men) Certain diagnosis of acute ITP in a child: • • Severe thrombocytopenia, signs of hemorrhage, normal RBC and WBC in CBC Severe thrombocytopenia, sudden hemorrhage, no family history Most acute cases will recover in about 3 weeks Chronic ITP is mostly in adults (women > men) PRIMARY HEMOSTASIS TESTING: PLT FUNCTION Sample collection and handling: critical • What are the consequences of: • Short draw • Specimen clot • Visible hemolysis • Lipemia or icterus • Tourniquet application >1 minute • Specimen storage at 1–6° C • Specimen storage at >25° C • For hemostasis studies? PLT TESTINGS • Anti-coag of choice • RI • Manual and automated counting • Size and MPV •Bleeding Time - original test of platelet function now replaced by: • • PFA-100 Analyzers o Automated platelet function testing Platelet Aggregometry studies o Measure how well PLTs aggregate together to form clots PFA-100 • Automated system for analyzing platelet aggregation • Citrated whole blood is aspirated at high shear rates through disposable test cartridges • Test cartridges contain two membranes: one coated collagen/epinephrin (CEPI) and the other one coated with collagen/ADP (CADP)and an aperture • Agonists induce platelet adhesion, activation, and aggregation • Time required for a PLT plug to occlude aperture is an indication of PLT function • Assessment for inherited, acquired, or drug-induced platelet dysfunction • Can be used as an initial screening test for patients with impaired primary hemostasis (VWD) ‒ Successful at detecting VWD • Monitors Desmopressin (DDAVP) therapy in pre-surgical patients • DDAVP - antidiuretic drug that releases VWF from the cells as a side effect in order to assist with any mucocutaneous bleeding • Assesses platelet dysfunction due to the effect of Aspirin (or efficacy of Aspirin therapy) PLT AGGREGOMETRY Measure how well PLTs aggregate together to form clots Sample procurement very important: • • Must NOT activate platelets Use larger bore needle Spin low to produce Platelet RICH Plasma (PRP) • • • Allow platelets to regain function- let sample sit for ~ 30 minutes Test within 4 hours of collection to avoid spontaneous in vitro platelet activation and loss of normal activity Chilling destroys PLT activity- samples held at 15-25ºC until tested OPTICAL AGGREGOMETER Testing Procedure PRP in a cuvette with a plasticized, magnetic stir-bar: • • Keeps platelets in suspension at 800-1200 rpm (gentle speed) Warm to 37C for 5 minutes Photometer directs light through cuvette to photodetector • • OD of PRP is high Baseline established = 0% li transmission Add agonist (PLT activator or aggregating agent) • Platelet shape change – increase in %T • Platelets start aggregating – increase in %T • Platelets form large aggregate – up to 100%T Storage Pool Defects- Dense Granules • • Prolonged Bleeding Time or abnormal PFA-100 testing Decreased platelet aggregation to some agonists • • • • Early monitoring using 4Ts Scoring System Enzyme Immunoassay Confirmatory PLT Activation Assay (Serotonin Release Assay) Rapid PLT agglutination immunoassay also available HIT Vascular Disorders • • • May have prolonged BT or abnormal PLT function testing Other coagulation tests are usually normal Physicians work to rule out other causes for bleeding Case 1 A 35-year-old woman noticed multiple pinpoint red spots and bruises on her arms and legs. The hematologist confirmed the presence of petechiae, purpura, and ecchymoses on her extremities and ordered a complete blood count, prothrombin time, and partial thromboplastin time. The platelet count was 35 × 109/L, the MPV was 13.2 fL, and the diameter of platelets on the Wrightstained peripheral blood film appeared to exceed 6 μm. Other CBC parameters and the coagulation parameters were within normal limits. A Wright-stained bone marrow aspirate smear revealed 10 to 12 small unlobulated megakaryocytes per low-power microscopic field. 1. Do these signs and symptoms indicate mucocutaneous (systemic) or anatomic bleeding? 2. What is the probable cause of the bleeding? 3. Does the patient’s bleeding result from altered platelet production in the bone marrow? 4. List the growth factors involved in recruiting megakaryocyte progenitors Case 2 A 55-year-old man comes to the emergency department with epistaxis. He reports that he has “bleeder’s disease” and has had multiple episodes of inflammatory hemarthroses. Physical examination reveals swollen, immobilized knees; mild jaundice; and an enlarged liver and spleen. Complete blood count results indicate that the patient is anemic and has thrombocytopenia with a platelet count of 74,400/μL. PT is within reference range and aPTT is 43 seconds. 1. What is the most likely diagnosis? 2. What treatment does the patient need? Case 3 A 19-year-old woman with a chief complaint of easy bruising, occasional mild nosebleeds, and heavy menstrual periods was examined by her physician. At the time of her examination, she had a few small bruises on her arms and legs, but no other findings. Initial laboratory data: normal PT and PTT, normal CBC. Detailed history: the patient’s bleeding problems occurred most frequently after aspirin ingestion. Her mother and one of her brothers also had some of the same symptoms. Blood was drawn for platelet function studies. Platelet aggregation tests indicated that although the response to ristocetin and ADP were near normal, arachidonic acidinduced aggregation was absent, epinephrine induced only primary aggregation, and collagen-induced aggregation was decreased (although a near-normal aggregation response could be obtained with a high collagen concentration). 1. What are three possible explanations for the test results? 2. Given the bleeding history in her family, which of the three explanations seems most likely? A quantitative test for adenosine triphosphate (ATP) release was performed using the firefly luciferin-luciferase bioluminescence assay. The result of this test showed a marked decrease in the amount of ATP released when platelets were stimulated with thrombin. 3. Based on the ATP release test results, what is the likely cause of the patient’s bleeding symptoms? Case 4 A 73-year-old Hispanic woman, who has had 4 children in the remote past, is hospitalized in the medical intensive care unit with end-stage liver disease secondary to cirrhosis as a result of chronic hepatitis B infection. Her clinical course has been complicated by recurrent massive abdominal ascites, episodes of spontaneous bacterial peritonitis, DIC, respiratory failure (she is now intubated, on a ventilator), and worsening renal failure (thought to be secondary to hepatorenal syndrome). Her platelet counts, which average in the low 20s × 106/μL, have not been associated with spontaneous bleeding. She is not on any medications that affect platelet function. Wanting to start hemodialysis, her physicians have requested interventional radiology (IR) to place a central line. An IR physician insists on a preprocedure platelet count of at least 50 × 106/μL before line placement. Despite platelet transfusions during the last 3 days, repeat platelet counts the next day remained at baseline. 1. What is the most likely cause of her thrombocytopenia? 2. What laboratory tests should be ordered next? 3. One doctor suggested that “specialized platelets” (e.g., crossmatched or HLA-matched components) might work better. Is this suggestion true?