Thermodynamics Problem Solutions: Saturated Water & Air Compression
advertisement
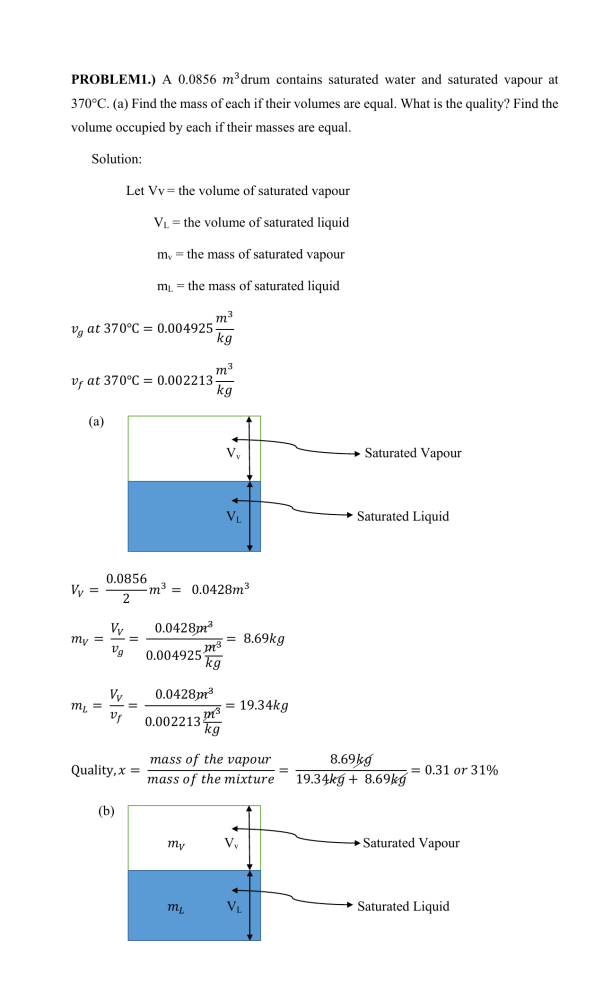
PROBLEM1.) A 0.0856 𝑚3 drum contains saturated water and saturated vapour at 370°C. (a) Find the mass of each if their volumes are equal. What is the quality? Find the volume occupied by each if their masses are equal. Solution: Let Vv = the volume of saturated vapour VL = the volume of saturated liquid mv = the mass of saturated vapour mL = the mass of saturated liquid 𝑚3 𝑣𝑔 𝑎𝑡 370℃ = 0.004925 𝑘𝑔 𝑚3 𝑣𝑓 𝑎𝑡 370℃ = 0.002213 𝑘𝑔 (a) Vv VL 𝑉𝑉 = Saturated Vapour Saturated Liquid 0.0856 3 𝑚 = 0.0428𝑚3 2 𝑉𝑉 𝑚𝑉 = = 𝑣𝑔 0.0428𝑚3 = 8.69𝑘𝑔 𝑚3 0.004925 𝑘𝑔 𝑉𝑉 𝑚𝐿 = = 𝑣𝑓 0.0428𝑚3 = 19.34𝑘𝑔 𝑚3 0.002213 𝑘𝑔 Quality, 𝑥 = 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑡ℎ𝑒 𝑣𝑎𝑝𝑜𝑢𝑟 8.69𝑘𝑔 = = 0.31 𝑜𝑟 31% 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑡ℎ𝑒 𝑚𝑖𝑥𝑡𝑢𝑟𝑒 19.34𝑘𝑔 + 8.69𝑘𝑔 (b) 𝑚𝑉 Vv 𝑚𝐿 VL Saturated Vapour Saturated Liquid 𝑚𝑉 = 𝑚𝐿 𝑉𝑉 + 𝑉𝐿 = 0.0856 𝑉𝑉 = 𝑚𝑉 𝑣𝑔 = 0.004925𝑚𝑉 𝑉𝐿 = 𝑚𝐿 𝑣𝑓 = 0.002213𝑚𝐿 Substituting in equation 2, 0.004925𝑚𝑉 + 0.002213𝑚𝐿 = 0.0856 0.004925𝑚𝑉 + 0.002213𝑚𝑉 = 0.0856 𝑚𝑉 = 11.9 𝑘𝑔 𝑚𝐿 = 11.9 𝑘𝑔 𝑚3 𝑉𝑉 = 𝑚𝑉 𝑣𝑔 = 11.9 𝑘𝑔 (0.004925 ) = 0.05905𝑚3 𝑘𝑔 𝑚3 𝑉𝐿 = 𝑚𝐿 𝑣𝑓 = 11.9 𝑘𝑔 (0.002213 ) = 0.02653𝑚3 𝑘𝑔 PROBLEM 2.) Air at 200 kPa, 30°C is contained in a cylinder/piston arrangement with initial volume 0.1 m3. The inside pressure balances ambient pressure of 100 kPa plus an externally imposed force that is proportional to V0.5. Now heat is transferred to the system to a final pressure of 225 kPa. Find the final temperature and the work done in the process. Solution: C.V. Air. This is a control mass. Use initial state and process to find T2 𝑃1 = 𝑃0 + 𝐶𝑉 0.5 200 = 100 + 𝐶(0.1)0.5 𝐶 = 316.23 225 = 100 + (316.23)𝑉2 0.5 𝑉2 = 0.156, 𝑚3 𝑃2 𝑉2 = 𝑚𝑅𝑇2 = 𝑇2 = ( 𝑃1 𝑉1 𝑇2 𝑇1 𝑃2 𝑉2 225(0.156)(303.15) = 532𝐾 = 258.9°𝐶 ) (𝑇1 ) = 𝑃1 𝑉1 200(0.1) 𝑊12 = ∫ 𝑃𝑑𝑉 = ∫(𝑃0 + 𝐶𝑉 0.5 )𝑑𝑉 𝑊12 = 𝑃0 (𝑉2 − 𝑉1 ) + 𝐶2 3 3 3 0.5 (316.23)(2) 2 𝑊12 = 100(0.156 − 0.1) + (0.156 − 0.12 ) = 11.9 𝑘𝐽 3






