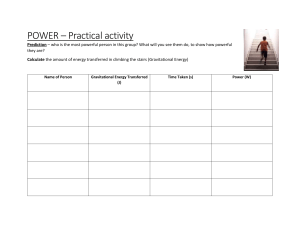
2012 Langer MIE experiements and simultaneous measurements...A varification of the ignition threshold Journal of Elec
advertisement

This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Journal of Electrostatics 70 (2012) 97e104 Contents lists available at SciVerse ScienceDirect Journal of Electrostatics journal homepage: www.elsevier.com/locate/elstat MIE experiments and simultaneous measurement of the transferred charge e A verification of the ignition threshold limits T. Langer*, G. Gramse, D. Möckel, U. von Pidoll, M. Beyer Physikalisch-Technische Bundesanstalt, Bundesallee 100, 38116 Braunschweig, Germany a r t i c l e i n f o a b s t r a c t Article history: Received 24 January 2011 Received in revised form 26 October 2011 Accepted 6 November 2011 Available online 17 November 2011 Using the apparatus for the determination of the MIE a wide series of experiments have been carried out in hydrogen/air, ethene/air, propane/air and acetone/air mixtures. The transferred charge as a criterion to judge the ignition potential is determined to verify the thresholds of transferred charge given in the standards. The stored charge in the capacitance before the discharge is compared to the transferred charge in the spark. The correlation of ignition energy and transferred charge is examined and the thresholds of the transferred charge are discussed. The MIE of the above-mentioned mixtures are reviewed taking into account the measurement uncertainty. Ó 2011 Elsevier B.V. All rights reserved. Keywords: Minimum ignition energy (MIE) Spark discharge Transferred charge Hand-held coulombmeter Ignition threshold Measurement uncertainty 1. Introduction The determination of the minimum ignition energy (MIE) using a capacitive spark discharge is well established in the literature as well as in standards [1e6]. Thereby, a capacitor with a defined capacitance is supplied by a defined voltage. Then the capacitor is discharged in a test vessel filled with a certain flammable gas/air mixture via an electrode configuration with a defined electrode diameter, electrode material and electrode gap width. The energy stored in the capacitor is calculated using the equation W ¼ 0:5$C$U 2 ; (1) where W is the energy, C is the capacitance and U is the voltage supplied to the capacitance. The energy of the spark discharge is assumed to have the amount of energy stored in the capacitance before the discharge. The MIE is determined by varying the capacitance, the charging voltage, the electrode diameter, the electrode gap width and the mixture composition in order to find the lowest energy necessary to ignite the given mixture. * Corresponding author. Tel.: þ49 531 592 3438; fax: þ49 531 592 3705. E-mail address: Tim.Langer@ptb.de (T. Langer). 0304-3886/$ e see front matter Ó 2011 Elsevier B.V. All rights reserved. doi:10.1016/j.elstat.2011.11.001 Based on MIE measurements, von Pidoll et al. [6] have calculated the charge stored on the capacitance in order to obtain an estimation of the transferred charge using the equation. Q ¼ C$U; (2) where Q is the charge. By correlating the MIE with the lowest transferred charge leading to ignition of the different mixtures, von Pidoll et al. [6] have suggested transferred charge thresholds in order to judge if electrostatic discharges are potentially incendive or not. These threshold values are now defined in the standards IEC 60079-0 and EN 13463-1 [3,4]. There, different gases, vapours and dusts are classified in explosion groups according to their hazards (I, IIA, IIB, IIC, III) [3,4]. For each explosion group, a maximum transferred charge has been defined which should ensure that a discharge with a transferred charge less than the defined maximum does not ignite the representatives of the specific group. In a recent study Langer et al. have found that these thresholds are sufficient concerning brush discharges regarding explosion groups IIA and IIC. But in case of explosion group IIB the achieved results were close to the defined threshold. It was concluded that the thresholds represent different levels of safety [7]. However, in [6], the transferred charge was not used for the definition of the transferred charge thresholds. In fact the charge stored in the capacitance before the discharge happened was taken. Since Chubb [8] and Walmsley [9] additionally stated that Author's personal copy 98 T. Langer et al. / Journal of Electrostatics 70 (2012) 97e104 depending on the used electrode radius, gap distance and charging voltage a transferred charge detected with unshielded probes might give wrong results e or more precisely an underestimation due to measured values being to low e it seems appropriate to first validate the thresholds in the standards by comparing both the charge stored before the discharge and the charge transferred in the discharge. Secondly, at the same time, the suitability of the method “transferred charge” using unshielded probes can be reviewed since the charging of the capacitance before the discharge occurs is well known using equation (2). This is in contrast to the latest verification of the thresholds using brush discharges [7] where the charging of an insulating surface cannot be determined that easily. Four different gases were examined e hydrogen as a representative of explosion group IIC, ethene for IIB and propane for IIA. Additionally, acetone was used since it has a higher minimum ignition energy compared with the other gases and, therefore, offers the opportunity to measure higher values of transferred charge. Performing the tests near the MIE of the mixtures is necessary to validate the transferred thresholds in the standards [3,4]. The aim of this work is to examine the correlation between the charge stored in the capacitance before the discharge Qc and the charge transferred in the discharge Qt. Furthermore, the dependence of the ignition energy and the transferred charge is studied. Based on the results, the thresholds of transferred charge and the suitability of the test method “transferred charge” using unshielded probes are discussed. Since the validation of the transferred charge threshold can only be reliably performed near the MIE of the certain mixtures, another objective of this work is to recheck the MIE of hydrogen, ethene, propane and acetone. Especially concerning propane and acetone, there have been some discussions in the recent past [10,11]. Furthermore, using the “Guide to the expression of uncertainty in measurement” [12], the received values are examined for the first time concerning the measurement uncertainty which has not been discussed in detail in the past. The considerations concerning the uncertainty should lead to an estimation as to how accurate the results given in the literature can be expected to be. This paper is structured as follows: section 2 contains the description of the test set-up and the experimental procedure. In section 3 the measurement uncertainty is discussed. The results and their discussion are presented in section 4. Finally, the resulting conclusions are given in section 5. 2. Test set-up and experimental procedure A schematic top view of the test set-up is given in Fig. 1. The test set-up consists of a metal vessel with a round base. Its inner diameter is 100 mm. The height is 85 mm. Therefore, the volume consists of approximately 670 cm3. An electrode configuration with an adjustable gap width and an electrode diameter is integrated in the vessel. Ball bearings were used as electrodes. The high voltage electrode is led insulated through the grounded vessel. A voltage in the range of several kV is given to a capacitor which is an air capacitor or just the capacitance of the electrode configuration together with the test set-up depending on the demanded size of the capacitance. The capacitance is measured using a calibrated capacitance bridge (Wayne-Kerr 4210). The voltage is delivered by a high voltage supply unit (F.u.G., HCN 140M-35000). The voltage is measured using a calibrated voltage divider (Spellman HVD 100, divider ratio 1:10008) and a voltage measuring device (Agilent 34410A). The voltage supply and the capacitance are connected in series via a resistor. Its resistance is e for all experiments e between 1 GU and 50 GU. The other electrode is grounded via a hand-held coulombmeter (Schnier Elektrostatik GmbH, Type HMG 11/02). Using this hand-held coulombmeter (HC) the transferred charge during a spark discharge can be measured. The measurement principle of the HC (see Fig. 2) is based on the discharge current which charges a capacitor with defined capacitance C. This leads to an increase in the voltage U at the capacitor. The voltage rise is measured internally by a processor which scans the voltage to find increasing or decreasing edges. The sampling rate is 100 kHz. The difference between the final and the initial value represents the voltage rise at the capacitor due to the transferred charge Qt during the discharge. The measurement result is calculated and given on a display. Fig. 1. Experimental set-up. Author's personal copy T. Langer et al. / Journal of Electrostatics 70 (2012) 97e104 99 The results presented here are based on several thousand discharge events. In fact, more than 3.500 spark discharges have been used for hydrogen/air, more than 5.000 for ethene/air, more than 12.000 for propane/air and more than 2.500 for acetone/air. 3. Measurement uncertainty Fig. 2. Schematic view of the hand-held microprocessor-operated coulombmeter. A UV radiation source is installed at the vessel in order to make spark discharges more reliable. The mixture is prepared using the partial pressure method [13]. Therefore, the vessel is evacuated. Thereby, pressure values lower than 2 mbar are reached with the test set-up. Afterwards the required amount of flammable gas is inserted in the vessel before the volume is filled with dried air (relative humidity 20%) until the ambient pressure is reached. The temperature during the experiments was 22 C 2 K. For the tests, the electrode configuration and the gap width are chosen. A defined charging voltage is given to the capacitance. Thereby, the voltage is close to the onset voltage for the formation of spark discharges for the given electrode configuration which has been observed in pretests. The transferred charge of each spark discharge is recorded with the HC. The time constant (s ¼ RC) of the capacitance and the series resistor was chosen to be more than 30 ms in order to prevent additional energy supply from the power supply during the spark discharge which is considerably shorter in time. In fact, the additional energy supply can be calculated using this equation W ¼ U$I$t ¼ U 2 $t=R (3) In order to obtain a worst case estimation, a voltage of 10,000 V, a resistance of 1 GU and a discharge duration of 100 ns are assumed. The average voltage used is lower than 10,000 V, the resistance higher and the discharge duration can be expected to be shorter than 100 ns. However, using these values, an additional energy supply of 10 nJ results. Therefore, the decoupling of the capacitance from the power supply using a high resistance is justified. The experiment runs until an ignition of the mixture is observed. The corresponding voltage is noted and the ignition energy is calculated using equation (1). Additionally, the transferred charge of the incendive spark is measured. Furthermore, the originally stored charge in the capacitor Qc can be calculated using equation (2). Reducing the ignition energy was attempted by varying the capacitance and the charging voltage, respectively. For the tests the flammable gas/air mixtures prepared were in the most ignitable range for hydrogen, ethene and propane according to [14], since this is the most hazardous situation one can examine in matters of explosion protection. Hence, a concentration of 22.0% hydrogen in air, 8.0% ethene in air and 5.2% propane in air was used for the experiments. For acetone, a concentration of 6.5% was used which is most ignitable according to [14]. Using hydrogen/air mixtures, an electrode configuration of a 15 mm diameter electrode and of a 2 mm diameter electrode was used for one part of the experiments. For the determination of the MIE of hydrogen/air mixtures a small capacitance was needed. Therefore, the capacitance of the electrode 15 mm/2 mm configuration together with the cable capacitance was sufficient. Thereby, the 15 mm electrode was at high voltage and the 2 mm electrode grounded. Later the configuration 2 mm/2 mm was tried too, which then led to the lowest results concerning the ignition energy. For the examination of the propane/air mixtures, a configuration of 1.5 mm (high voltage) and 2 mm electrodes was used. For all other mixtures, a configuration of two 2 mm diameter electrodes was used. Before presenting the results, the consideration of the measurement uncertainty is explained. The expanded measurement uncertainty is determined according to the internationally accepted “Guide to the expression of uncertainty in measurement” [12]. The important parameters which influence the uncertainty of the ignition energy and the calculated charge are the voltage U and the capacitance C. Considering the voltage measurement, an accuracy of 6 104 results when taking into account the uncertainties of the calibration of the voltage divider and the voltage measuring device. The uncertainty in the determination of the capacitance was estimated to be 0.5 pF which results mainly from the zero point adjustment before the actual measurement is performed. This uncertainty is much more than what the capacitance bridge contributes and can be ascribed to the problem of the determination of small capacitances (in the range of a few pF) e.g. due to the influence of stray capacitances. For all uncertainties given in this work, the coverage factor k is 2.0. By multiplying the measurement uncertainty with the coverage factor, it is assured that all measurement results have a probability of 95% of being within the resulting expanded measurement interval. Considering the ignition energy according to equation (1) and the calculated charge stored on the capacitance according to equation (2), it is found that the influence of the uncertainty in C is much higher than the uncertainty in U. The uncertainty consists in all cases to more than 99.8% of the contribution in the uncertainty in C. The measuring range, in which the HC can be used adequately, is between approximately 10 nC and 200 nC. In [15] Langer et al. discussed the relevant influences on the measurement uncertainty of the HC. This knowledge was then used to develop a calibration method for the HC. According to this calibration method, the HC has a deviation DQ between the measured transferred charge Qt and the reference value Qr. Moreover, an expanded measurement uncertainty was determined according to [12] with a coverage factor k ¼ 2.0. Its dependence on the amount of transferred charge is as follows: Table 1. The measuring results can be corrected by considering the deviation in dependence of the measuring interval of the HC. The expanded uncertainty remains. For all results presented here, the deviation is already taken into account. The electrode gap width can be adjusted with an accuracy of 0.1 mm. The mixture compositions have an accuracy of 0.1%. 4. Results and discussion The HC did not measure the transferred charge Qt of every spark. The probability of measuring the transferred charge during the Table 1 Results of the hand-held coulombmeter calibration. Qr/nC Qt/nC DQ/nC Expanded measurement uncertainty/nC k 12.4 30.6 60.9 91.2 121.5 151.9 182.2 9.8 28.7 59.4 89.9 119.9 150.3 181.4 2.6 1.9 1.5 1.3 1.6 1.6 0.8 0.7 1.1 1.8 2.4 3.4 4.2 5.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 Author's personal copy 100 T. Langer et al. / Journal of Electrostatics 70 (2012) 97e104 spark occurrence is lower when getting near the minimum trigger level of approximately 10 nC as is necessary in case of hydrogen/air mixtures. In case of hydrogen/air mixtures even a discrepancy between the two different electrode configurations used can be seen. By using the 2 mm/2 mm configuration, the capacitance was reduced again compared to the 15 mm/2 mm configuration. Therefore, the amount of charge stored on the capacitance before the spark occurred decreased too, resulting in an even lower measurement probability of Qt. For the other mixtures with transferred charges higher than the trigger level, the frequency of measuring Qt was more than 80% as can be seen in Table 2. Only the experiments with an ignition of the mixture are considered here. Non-triggering of the HC has a minor part at this result. Mainly the manual resetting between two measurements with the HC caused a probability lower than 95% due to occurring sparks before the HC was reset. In Figs. 3e6 the correlation between the transferred charge Qt and the calculated charge Qc is presented for the four different examined mixtures. The “ignition” points represent the experiments in which a spark ignited the mixture and the HC measured the transferred charge Qt. For the clearness of the figures the expanded measurement uncertainty in the calculated charge Qc is only shown for the lowest and highest Qc. However, for all other Qc, it is in the same range. In all figures a fit of Qt ¼ Qc is given. In reality the transferred charge cannot be expected to match the calculated charge as there is always some residual charge on the capacitance after a spark discharge [16]. In [6] a 5% residual charge is given as an estimation. In [17] a residual charge of up to 50% was found. Furthermore, losses through corona cannot be excluded even though the capacitance is continuously charged by the supplied voltage since the time constant is quite high. Therefore, all transferred charges Qt should be lower than the calculated charge Qc and, thus, should lie under the fitted line. Taking the expanded measurement uncertainty into account, almost every measurement not depending on the mixture showed the expected Qt Qc. Furthermore, considering propane/air (Fig. 5) and acetone/air mixtures (Fig. 6) it can be seen that the ratio Qt/Qc decreases for higher Qc compared to hydrogen/air (Fig. 3) and ethene/air mixtures (Fig. 4). The average charging voltage was about 3 kV for hydrogen/air, 6 kV for ethene/ air and 9 kV for propane/air and acetone/air mixtures. Therefore, higher charging voltages could lead to more corona losses, for instance, resulting in a lower transferred charge Qt. Furthermore, higher charging voltages correspond to higher gap distances. Considering the results of Bane [17], there seems to be a dependence of the electrode gap width leading to higher residual charges at higher gap distances. This is not understood so far and should be studied in the future. In summary, it can be stated that the HC measured reasonable values. The theoretical work of Walmsley [9] investigating the effect of induced charge on the measurement of the transferred charge during brush and spark discharges when using unshielded probes showed that for the electrode radii, gap Fig. 3. Correlation between transferred charge Qt and calculated charge Qc for ignition in a 22.0% hydrogen/air mixture. distances and added capacitances used in the tests of this work induced charge would be of negligible amount and therefore, would not significantly influence the measurement of the transferred charge in this test set-up. This is in accordance to the results presented in Figs. 3e6. In the following, the criterion of the transferred charge Qt in order to judge the safety of products and items is reviewed based on the values presented in Figs. 3e6. Therefore, the transferred charge Qt in dependence of the ignition energy (calculated using equation (1)) for the observed ignitions is presented in Figs. 7e10 for the different mixtures. Thereby, the results are from different electrode gap widths. The criterion of transferred charge is based on the MIE values received in former studies [6] which are strongly connected with finding the optimum in all important parameters (capacitance, charging voltage, electrode diameter, electrode gap width, mixture composition). However, the criterion of transferred charge can only be suitable for judging the safety of test items when for all ignition energies e depending on the mixture e a transferred charge higher than the threshold value given in [3,4] is found. Table 2 Frequency of measuring the transferred charge in case of an ignition. Mixture Measurement of Qt No recorded measurement of Qt Probability of measurement/% Hydrogen/aira Hydrogen/airb Ethene/air Propane/air Acetone/air 3 12 27 69 34 15 12 3 8 8 17 50 90 90 81 a b Electrode configuration 2 mm/2 mm. Electrode configuration 15 mm/2 mm. Fig. 4. Correlation between transferred charge Qt and calculated charge Qc for ignition in an 8.0% ethene/air mixture. Author's personal copy T. Langer et al. / Journal of Electrostatics 70 (2012) 97e104 101 Fig. 5. Correlation between transferred charge Qt and calculated charge Qc for ignition in a 5.2% propane/air mixture. Fig. 7. Transferred charge Qt in dependence of the ignition energy for a 22.0% hydrogen/air mixture. In Figs. 8e10, the threshold value according to the explosion group given in the standard is drawn. Additionally, the hatched box represents the interval of ignition energy which is under the MIE given in [18]. For acetone in Fig. 11, the value of minimum transferred charge of 127 nC given in [6] is used. The MIE value of 0.55 mJ is also taken from [18]. In case of hydrogen/air mixtures which represent explosion group IIC (Fig. 7), all observed transferred charge values are over the threshold of 10 nC given in [3,4]. The lowest transferred charge igniting the mixture was 10.8 nC 0.7 nC corresponding to an ignition energy of 25.1 mJ 4.0 mJ. The transferred charge was not measured for the lowest ignition energy. However, taking the measurement uncertainty into account and the general difficulty in measuring transferred charges near the trigger level of the HC, the received results fulfil the requirements concerning the criterion of transferred charge since all received measurement results are over the threshold of 10 nC. In case of ethene/air mixtures which represent explosion group IIB (Fig. 8), sparks with a transferred charge less than the threshold of 30 nC ignited the mixture. Furthermore, ignition energies lower than the MIE are observed. The lowest transferred charge igniting the mixture was 24.1 nC 1.1 nC corresponding to an ignition energy of 91.1 mJ 9.9 mJ. A recalculation of the values which formed the base of the given threshold of IIB [6] in the standards showed that even the minimum transferred charge Qmin based on the voltage and capacitance given in [6] does not lead to a Qmin of 32.0 nC but to 30.2 nC. For propane/air mixtures as a representative of explosion group IIA (Fig. 9), the lowest transferred charge igniting the mixture was 66.1 nC 1.8 nC corresponding to an ignition energy of 0.382 mJ 0.025 mJ. However, the existing threshold seems adequate since all received transferred charges are over the threshold of 60 nC. In case of acetone/air mixtures (Fig. 10), transferred charges lower than the value given in [6] were observed. The lowest transferred charge igniting the mixture corresponds to the MIE measured in out tests. However, ignition energies lower than the MIE given in [18] were found. Therefore, for acetone, a revision of the MIE might be advisable. Since the results presented here are for incendive sparks only, these tests form an excellent base for the revision of the thresholds given in the standard [3,4] and the literature [6]. As could been Fig. 6. Correlation between transferred charge Qt and calculated charge Qc for ignition in a 6.5% acetone/air mixture. Fig. 8. Transferred charge Qt in dependence of the ignition energy for an 8.0% ethene/ air mixture. Author's personal copy 102 T. Langer et al. / Journal of Electrostatics 70 (2012) 97e104 Fig. 9. Transferred charge Qt in dependence of the ignition energy for a 5.2% propane/ air mixture. Fig. 11. Ignition energy in dependence of the electrode gap width using a 22.0% hydrogen/air mixture, determination of the MIE. seen, the criterion of transferred charge is suitable but the thresholds represent different levels of safety. In case of explosion group IIB, the achieved values of transferred charge leading to ignition are close to the threshold whereas in case of explosion group IIC, the gap between the achieved results and the threshold is somewhat bigger. In the last part of this paper, the MIE values of hydrogen, ethene, propane and acetone/air mixtures given in the literature e.g [1,2,6,10,11,14,18e20]. are reviewed. Thereby, especially the measurement uncertainty is the focus of this study. In contrast to the data presented above, all observed ignitions are taken into account. Thus also the experiments in which the HC non-triggered the discharges are used for this study, since the transferred charge is not necessary to obtain the ignition energy. The ignition energy is once more calculated using equation (1). The MIE of hydrogen was recently verified by Ono et al. [20]. They observed a MIE of 17 mJ for a hydrogen/air mixture with a 22%e26% hydrogen fraction. This is in accordance with the value of 17 mJ given in [18]. In [2] 19 mJ and in [14] 16 mJ are given. As mentioned before two different electrode configurations were used for the 22.0% hydrogen/air mixture (Fig. 11). This is indicated with different symbols in the figure. The MIE determined in our test was observed at d ¼ 0.5 mm with two 2 mm electrodes at a voltage of 2.80 kV and a capacitance of 4.41 pF. Its value is 17.3 mJ 2.3 mJ and agrees well with the values between 16 mJ and 19 mJ given in [2,14,18,20]. For this value no transferred charge was measured. The corresponding calculated charge is 12.4 nC 1.6 nC. Concerning 8.0% ethene/air mixtures (Fig. 12), an MIE of 80.8 mJ 9.7 mJ was found at a gap width of 1.2 mm using two 2 mm electrodes at a voltage of 5.80 kV and a capacitance of 4.80 pF. As described above, the biggest influence with respect to the expanded measurement uncertainty originates from the determination of the capacitance. The transferred charge was 26.9 nC 1.1 nC, the calculated charge 27.9 nC 3.3 nC. Regarding the measurement uncertainty, the MIE is the same as the value of 82 mJ given in [18]. The MIE of propane/air mixtures (Fig. 13) is in discussion [10,19] since the value of 0.25 mJ given by Lewis and von Elbe [2] and in [14] seems to be quite low. In [18] 0.24 mJ is given. Its origin could not be verified. For 5.2% propane in air, a 1.5 mm/2.0 mm electrode configuration and a gap width of 1.7 mm was used. An MIE of 0.330 mJ 0.020 mJ was found using a voltage of 8.32 kV and Fig. 10. Transferred charge Qt in dependence of the ignition energy for a 6.5% acetone/ air mixture. Fig. 12. Ignition energy in dependence of the electrode gap width using an 8.0% ethene/air mixture, determination of the MIE. Author's personal copy T. Langer et al. / Journal of Electrostatics 70 (2012) 97e104 103 Table 3 Overview of achieved results concerning the MIE determination. Fig. 13. Ignition energy in dependence of the electrode gap width using a 5.2% propane/air mixture, determination of the MIE. a capacitance of 9.53 pF. The corresponding value of transferred charge was 66.9 nC 1.8 nC, the calculated charge 79.3 nC 4.8 nC. It should be pointed out that the results for propane are based on more than 12.000 spark discharges. The MIE is over 30% higher than the values given by Lewis and von Elbe [2] and Brandes and Möller [18]. On the other hand, it is significantly lower than the recently published value of 0.46 mJ [10] and the value of 0.48 mJ given by Moorhouse et al. [19]. The value given by Moorhouse was measured in a slightly different mixture compared to the mixture used for this work (stoichiometric ratio 1.30 instead of 1.25 in this work). The gap width is not given in detail but is between 2.3 mm and 2.8 mm in the study of Moorhouse. Moreover, the used pressure was only 0.75 atm. Eckhoff et al. [10] used a gap width of 2.0 mm and two 1.6 mm flanged electrodes. The mixture was 5.2% propane in air as used for this work as well. Concerning 6.5% acetone/air mixtures (Fig. 14) the MIE observed in our tests was 0.499 mJ 0.018 mJ with an electrode gap of 1.9 mm and two 2.0 mm electrodes at a voltage of 7.90 kV and a capacitance of 16.00 pF. This value is about 10% lower than the value of 0.550 mJ given in [18]. In [11] it was criticised that for the Fig. 14. Ignition energy in dependence of the electrode gap width using a 6.5% acetone/air mixture, determination of the MIE. Mixture Qt at MIE/nC Expanded measurement uncertainty/nC MIE Expanded measurement uncertainty 22.0% hydrogen/air 8.0% ethene/air 5.2% propane/air 6.5% acetone/air e 26.9 66.9 112.8 e 1.1 1.8 3.4 17.3 mJ 80.8 mJ 0.330 mJ 0.499 mJ 2.3 mJ 9.7 mJ 0.020 mJ 0.018 mJ determination of the MIE of acetone/air mixtures often a stochiometric and not the most ignitable mixture is used leading to too high MIE values. The corresponding transferred charge is 112.8 nC 3.4 nC, the calculated charge 126.4 nC 4.6 nC. The transferred charge measured is more than 10% lower than the value given in [6]. In summary especially concerning the determination of MIE and their corresponding transferred charge, it seems essential to give the corresponding measurement uncertainties in order to compare the achieved results. The measurement of the capacitance seems to be the main challenge in this context. In Table 3 all achieved results are given for an overview. 5. Conclusions Using the apparatus commonly utilized for the determination of the minimum ignition energy (MIE), a wide series of experiments based on several thousand discharge events have been carried out in hydrogen/air, ethene/air, propane/air and acetone/air mixtures. Thereby, the transferred charge as a criterion to judge the ignition potential is determined to verify the thresholds of transferred charge given in the standards IEC 60079-0 and EN 13463-1. The stored charge in the capacitance before the spark discharge is compared to the transferred charge in the spark. The measurement of the transferred charge performed with a hand-held coulombmeter using an unshielded probe gave reasonable values for all examined mixtures. It was found that the transferred charge is less than the stored charge. However, this difference can be explained by a residual charge on the capacitance or corona, respectively. For higher gap distances or higher charging voltages, respectively, a decreasing ratio of the transferred charge to the stored charge was observed. Furthermore, the correlation of the ignition energy and the transferred charge is examined. Based on these results the thresholds of the transferred charge and the suitability of the method “transferred charge” are discussed. In case of ethene/air mixtures (explosion group IIB) a minimum transferred charge of 24.1 nC 1.1 nC led to ignition which is lower than the current threshold of 30 nC. Moreover, a lower transferred charge igniting acetone/air mixtures than given in the literature was found (112.8 nC 3.4 nC compared to 127 nC). For hydrogen/air and propane/air mixtures the lowest transferred charge leading to ignition was higher than the given threshold. In general, the lowest transferred charge igniting the mixture does not necessarily correspond to the MIE of the mixture. All in all, the criterion of the transferred charge seems suitable but the thresholds of the different explosion groups represent different levels of safety. The MIE values of the above-mentioned mixtures are reviewed taking into account the measurement uncertainty according to the “Guide to the expression of uncertainty in measurement”. For hydrogen/air mixtures, a value of 17.3 mJ 2.3 mJ was achieved which agrees well with the literature. The MIE of ethene/air mixtures was determined to be 80.8 mJ 9.7 mJ which matches well with the given value of 82 nC in the literature. The MIE of propane/ Author's personal copy 104 T. Langer et al. / Journal of Electrostatics 70 (2012) 97e104 air mixtures has been in discussion during the past few years. An MIE of 0.330 mJ 0.020 mJ was achieved which is less than the recently published values by Eckhoff et al. and Moorhouse et al. of 0.46 mJ and 0.48 mJ, respectively, but significantly more than the 0.24 mJ and 0.25 mJ given by Brandes and Möller and Lewis and von Elbe, respectively. The MIE of acetone/air mixtures was 0.499 mJ 0.018 mJ which is lower than the value of 0.550 mJ given in the literature. The main part of the measurement uncertainty results from the determination of the capacitance (setting the zero point). More work is planned in the future concerning the determination of MIE values especially regarding statistics along with the uncertainty. References [1] H.R. Calcote, et al., Spark ignition, effect of molecular structure, Ind. Engineering Chemistry 44 (1952) 2656. [2] B. Lewis, G. von Elbe, Combustion, Flames and Explosions of Gases, third ed. Academic Press INC, London, 1987. [3] International Standard IEC 60079-0 Ed 5.0: Explosive Atmospheres e Part 0: Equipment e General Requirements, 2007. [4] European Standard EN 13463-1: Non-electrical Equipment for Use in Potentially Explosive Atmospheres e Part 1: Basic Method and Requirements, 2007. [5] ASTM: Standard Test Method for Minimum Ignition Energy and Quenching Distance in Gaseous Mixtures. Designation E 582e07. ASTM Int., 2007. [6] U. von Pidoll, et al., Determining the incendivity of electrostatic discharges without explosive gas mixtures, IEEE Trans. Ind. Appl. 40 (2004) 1467. [7] T. Langer, et al., Transferred charge of brush discharges in explosive gas atmospheres e a verification of the ignition threshold limits, J. Electrostat. 69 (2011) 200e205. [8] J.N. Chubb, Measurement of charge transfer in electrostatic discharges, J. Electrostat. 64 (2006) 321e325. [9] H.L. Walmsley, Induced-charge errors in charge-transfer measurement: calculations for sparks and additional brush-discharge geometries, J. Electrostat. 69 (2011) 79e86. [10] R.K. Eckhoff, et al., On the minimum ignition energy (MIE) for propane/air, J. Hazard. Mat. 175 (2010) 293e297. [11] L.G. Britton, Avoiding Static Ignition Hazards in Chemical Operations. Troll Assoc Inc., New York, 1999. [12] ISO/IEC Guide 98-3, Uncertainty of Measurement e Part 3: Guide to the Expression of Uncertainty in Measurement (2008) ISBN 92-67-10188.9, Geneva, Switzerland. [13] H. Krämer, MIE of Flame-Retardant Chlorinated Hydrocarbons (In German). PTB Bericht, PTB-W-35, Braunschweig, Germany, 1988. [14] M. Hattwig, H. Steen, Handbook of Explosion Prevention and Protection. Wiley-VCH, 2004. [15] T. Langer, et al., Metrological characterization of electrostatic discharges (in German), Technisches Messen 75 (2008) 515e524. [16] T. Langer, diploma thesis, TU Braunschweig, 2007. [17] S.P.N. Bane, Spark ignition: experimental and numerical investigation with application in aviation safety, PhD thesis, California Institute of Technology, Pasadena, California, 2010. [18] E. Brandes, W. Möller, Safety Characterstic Data e Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag N.W. Verlag für neue Wissenschaft, Bremerhaven, 2008. [19] J. Moorhouse, et al., An investigation of the minimum ignition energies of some C1 to C7 hydrocarbons, Comb. Flame 23 (1974) 203e213. [20] Ono, et al., Minimum ignition energy of hydrogeneair mixture: effects of humidity and spark duration, J. Electrostat. 65 (2007) 87e93.